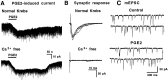Direct activation of rat spinal dorsal horn neurons by prostaglandin E2 - PubMed (original) (raw)
Direct activation of rat spinal dorsal horn neurons by prostaglandin E2
H Baba et al. J Neurosci. 2001.
Abstract
Whole-cell patch-clamp and intracellular recording techniques have been used to study the action of prostaglandin E2 (PGE2) on neurons in adult rat transverse spinal cord slices. Bath-applied PGE2 (1-20 microm) induced an inward current or membrane depolarization in the majority of deep dorsal horn neurons (laminas III-VI; 83 of 139 cells), but only in a minority of lamina II neurons (6 of 53 cells). PGE2 alone never elicited spontaneous action potentials; however, it did convert subthreshold EPSPs to suprathreshold, leading to action potential generation. PGE2-induced inward currents were unaffected by perfusion with either a Ca(2+)-free/high Mg(2+) (5 mm) solution or tetrodotoxin (1 microm), indicating a direct postsynaptic action. Both 17-phenyl trinor prostaglandin E2 (an EP1 agonist) and sulprostone (an EP3 agonist) had little effect on membrane current, whereas butaprost methyl ester (an EP2 agonist) mimicked the effect of PGE2. Depolarizing responses to PGE2 were associated with a decrease in input resistance, and the amplitude of inward current was decreased as the holding potential was depolarized. PGE2-induced inward currents were reduced by substitution of extracellular Na(+) with N-methyl-d-glucamine and inhibited by flufenamic acid (50-200 microm), which is compatible with activation of a nonselective cation channel. These results suggest that PGE2, acting via an EP2-like receptor, directly depolarizes spinal neurons. Moreover, these findings imply an involvement of spinal cord-generated prostanoids in modulating sensory processing through an alteration in dorsal horn neuronal excitability.
Figures
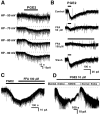
Fig. 1.
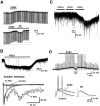
Effect of PGE2 on membrane properties of deep dorsal horn neurons. A, Depolarizing response produced by bath application of PGE2 (10 μ
m
) recorded intracellularly in current-clamp mode in the presence of TTX (1 μ
m
). Downward deflections are electrotonic voltage transients elicited by hyperpolarizing current pulses (amplitude, 0.15 nA; duration, 400 msec) to measure membrane input resistance (_R_in). The PGE2-induced depolarization was associated with a decreased_R_in. When the neuron was manually clamped (DC) to the resting membrane potential, the reduction in_R_in was unaffected. Therefore, the decreased_R_in was not caused by activation of voltage-gated channels. B, Blind whole-cell patch-clamp recording of a PGE2 (5 μ
m
)-induced inward current recorded in the presence of TTX (1 μ
m
) from a neuron voltage clamped to −70 mV. This neuron was recorded from a slice with an attached dorsal root, permitting determination of the type of afferent input. Neurons with both A- and C-fiber inputs (bottom) responded to PGE2 with larger inward currents than neurons with purely A-fiber input. C, Both PGF2α (10 μ
m
) and PGD2 (10 μ
m
) were without effect in this PGE2 (10 μ
m
)-responsive neuron.D, Intracellular recording from a deep dorsal horn neuron. Top, Bath-applied PGE2 (10 μ
m
) elicited a membrane depolarization. At the resting membrane potential, dorsal root stimulation at A-fiber intensity (30 μA, 0.05 msec, 0.5 Hz) elicited subthreshold EPSPs. During the PGE2-induced depolarization, evoked EPSPs reached action potential threshold. Action potentials are indicated by dots. When the neuron was manually clamped (DC) to the resting membrane potential, action potentials disappeared. Bottom, EPSPs and action potentials are shown on an expanded time scale. Note that the amplitudes of EPSPs during DC are not significantly different from control, indicating that the generation of action potentials is not caused by the augmentation of transmitter release from presynaptic terminals. Three consecutive traces are superimposed in each stimulating condition.
Fig. 2.
PGE2 acts postsynaptically. A, Whole-cell patch-clamp recording of a PGE2 (5 μ
m
)-evoked inward current in a deep dorsal horn neuron with only A-fiber input. The PGE2-induced inward current was not blocked by perfusion with the Ca2+-free/high Mg2+ (5 m
m
) Krebs' solution. B, In contrast, dorsal root-evoked monosynaptic EPSCs were completely abolished by perfusion with the Ca2+-free/high Mg2+solution. Traces in A and_B_ are from the same neuron. C, PGE2 did not affect the frequency of miniature EPSCs. The frequencies of miniature EPSCs before and during application of PGE2 were 46.2 and 44.3 Hz, respectively. This neuron responded to PGE2 with an inward current (see Fig. 1_B_).
Fig. 3.
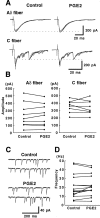
PGE2 has no effect on excitatory transmitter release in the SG (lamina II). A, The amplitude of Aδ-fiber (top traces) and C-fiber (bottom traces)-evoked monosynaptic EPSCs was not affected by PGE2 (10 μ
m
). Five consecutive traces are superimposed for each condition. They were averaged, and the difference between baseline and peak current was measured. B, Summary of the PGE2 (10 μ
m
) effects on the amplitude of dorsal root-evoked EPSCs. PGE2 did not significantly alter the amplitude of either Aδ- or C-fiber-evoked EPSCs in the SG (Aδ-fiber EPSC, p = 0.79, n = 9; C-fiber EPSC, p = 0.78, n = 8; paired_t_ test). The amplitudes of evoked EPSCs were measured 5–10 min after the start of PGE2 application. C, Miniature EPSCs recorded from a SG neuron in the presence of TTX (1 μ
m
). The frequencies of mEPSCs were 22.6 and 22.2 Hz before and during application of PGE2, respectively. D, The effect of PGE2 (10 μ
m
) on the frequency of mEPSCs. PGE2 did not significantly increase the frequency of mEPSCs in 14 of 15 SG neurons studied (p = 0.23, paired_t_ test). The frequency of mEPSCs was counted 5–10 min after the start of PGE2 application. All data were recorded at −70 mV.
Fig. 4.
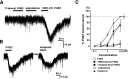
Effects of EP receptor agonists on membrane currents in deep dorsal horn neurons. A, An EP1 receptor agonist, 17-phenyl trinor prostaglandin E2 (10 μ
m
), and an EP3 receptor agonist, sulprostone (10 μ
m
), had no effect on membrane currents in neurons that responded to the nonselective EP receptor agonist, 19(R)-hydroxy prostaglandin E2 (10 μ
m
). B, The selective EP2 receptor agonist, butaprost methyl ester (10 μ
m
), induced an inward current similar to that produced by PGE2 (10 μ
m
). C, Comparison of the effects of the different EP agonists. Points represent the mean ± SD of the percentage maximum response to PGE2 (10 μ
m
).n = 3–6 in each group. EC50 values are 1.5 μ
m
for PGE2, 2.3 μ
m
for 19(R)-hydroxy prostaglandin E2, and 3.1 μ
m
for butaprost methyl ester.
Fig. 5.
Effects of membrane potential, flufenamic acid (FFA), and_N-_methyl-
d
-glucamine (NMDG) on PGE2-induced inward currents. A, This deep dorsal horn neuron was voltage clamped to different holding potentials, and PGE2 (10 μ
m
, 60 sec)-induced inward currents were recorded. Note that responses become smaller as the membrane potential is depolarized. HP, Holding potential.B, Bath-applied PGE2 (5 μ
m
) elicited an inward current in another neuron, as recorded by the whole-cell patch-clamp technique. FFA (50 μ
m)
partially blocked the PGE2-induced inward current. Increasing the concentration of FFA to 100 μ
m
almost completely blocked the PGE2-induced current, in a reversible fashion. C, Whole-cell patch-clamp recording reveals that application of FFA (100 μ
m
) during the PGE2 (5 μ
m
)-induced inward current reversed the effect of PGE2. D, Effect of decreasing extracellular Na+ on PGE2-induced inward currents. In normal Krebs' solution, bath-applied PGE2 (10 μ
m
) induced an inward current. Changing the perfusion solution to a low Na+ solution, in which Na+ was replaced with NMDG+ (a membrane impermeable cation), reversed the PGE2-induced inward current nearly to control values. Returning to normal Krebs' solution restored the PGE2-induced inward current. The amplitudes of spontaneous EPSCs are truncated. Neurons were voltage clamped to −70 mV in_B_–D.
Similar articles
- Role of prostaglandin receptor subtype EP1 in prostaglandin E2-induced nociceptive transmission in the rat spinal dorsal horn.
Nakayama Y, Omote K, Kawamata T, Namiki A. Nakayama Y, et al. Brain Res. 2004 Jun 4;1010(1-2):62-8. doi: 10.1016/j.brainres.2004.03.002. Brain Res. 2004. PMID: 15126118 - Changes in the effect of spinal prostaglandin E2 during inflammation: prostaglandin E (EP1-EP4) receptors in spinal nociceptive processing of input from the normal or inflamed knee joint.
Bär KJ, Natura G, Telleria-Diaz A, Teschner P, Vogel R, Vasquez E, Schaible HG, Ebersberger A. Bär KJ, et al. J Neurosci. 2004 Jan 21;24(3):642-51. doi: 10.1523/JNEUROSCI.0882-03.2004. J Neurosci. 2004. PMID: 14736850 Free PMC article. - Actions of prostaglandin E2 on rat supraoptic neurones.
Sutarmo Setiadji V, Shibuya I, Kabashima N, Ibrahim N, Harayama N, Ueta Y, Yamashita H. Sutarmo Setiadji V, et al. J Neuroendocrinol. 1998 Dec;10(12):927-36. doi: 10.1046/j.1365-2826.1998.00282.x. J Neuroendocrinol. 1998. PMID: 9870750 - Prostaglandin E2-induced modification of tetrodotoxin-resistant Na+ currents involves activation of both EP2 and EP4 receptors in neonatal rat nodose ganglion neurones.
Matsumoto S, Ikeda M, Yoshida S, Tanimoto T, Takeda M, Nasu M. Matsumoto S, et al. Br J Pharmacol. 2005 Jun;145(4):503-13. doi: 10.1038/sj.bjp.0706212. Br J Pharmacol. 2005. PMID: 15821755 Free PMC article. - Direct excitation of deep dorsal horn neurones in the rat spinal cord by the activation of postsynaptic P2X receptors.
Shiokawa H, Nakatsuka T, Furue H, Tsuda M, Katafuchi T, Inoue K, Yoshimura M. Shiokawa H, et al. J Physiol. 2006 Jun 15;573(Pt 3):753-63. doi: 10.1113/jphysiol.2006.108613. Epub 2006 Apr 13. J Physiol. 2006. PMID: 16613873 Free PMC article.
Cited by
- Repeated administration of a flavonoid-based formulated extract from citrus peels significantly reduces peripheral inflammation-induced pain in the rat.
Iannitti T, Di Cerbo A, Loschi AR, Rea S, Suzawa M, Morales-Medina JC. Iannitti T, et al. Food Sci Nutr. 2020 May 20;8(7):3173-3180. doi: 10.1002/fsn3.1566. eCollection 2020 Jul. Food Sci Nutr. 2020. PMID: 32724582 Free PMC article. - The absence of the leukotriene B4 receptor BLT1 attenuates peripheral inflammation and spinal nociceptive processing following intraplantar formalin injury.
Asahara M, Ito N, Yokomizo T, Nakamura M, Shimizu T, Yamada Y. Asahara M, et al. Mol Pain. 2015 Mar 12;11:11. doi: 10.1186/s12990-015-0010-9. Mol Pain. 2015. PMID: 25889478 Free PMC article. - Targeting inflammation as a treatment modality for neuropathic pain in spinal cord injury: a randomized clinical trial.
Allison DJ, Thomas A, Beaudry K, Ditor DS. Allison DJ, et al. J Neuroinflammation. 2016 Jun 17;13(1):152. doi: 10.1186/s12974-016-0625-4. J Neuroinflammation. 2016. PMID: 27316678 Free PMC article. Clinical Trial. - Central sensitization: a generator of pain hypersensitivity by central neural plasticity.
Latremoliere A, Woolf CJ. Latremoliere A, et al. J Pain. 2009 Sep;10(9):895-926. doi: 10.1016/j.jpain.2009.06.012. J Pain. 2009. PMID: 19712899 Free PMC article. Review. - [Pain therapy with antipyretic analgesics].
Hinz B, Brune K. Hinz B, et al. Orthopade. 2007 Jan;36(1):23-31. doi: 10.1007/s00132-006-1024-9. Orthopade. 2007. PMID: 17171384 Review. German.
References
- Beiche F, Scheuerer S, Brune K, Geisslinger G, Goppelt-Struebe M. Up-regulation of cyclooxygenase-2 mRNA in the rat spinal cord following peripheral inflammation. FEBS Lett. 1996;390:165–169. - PubMed
- Beiche F, Brune K, Geisslinger G, Goppelt-Struebe M. Expression of cyclooxygenase isoforms in the rat spinal cord and their regulation during adjuvant-induced arthritis. Inflamm Res. 1998;47:482–487. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous