Decreased nuclear beta-catenin, tau hyperphosphorylation and neurodegeneration in GSK-3beta conditional transgenic mice - PubMed (original) (raw)
Decreased nuclear beta-catenin, tau hyperphosphorylation and neurodegeneration in GSK-3beta conditional transgenic mice
J J Lucas et al. EMBO J. 2001.
Abstract
Glycogen synthase kinase-3beta (GSK-3beta) has been postulated to mediate Alzheimer's disease tau hyperphosphorylation, beta-amyloid-induced neurotoxicity and presenilin-1 mutation pathogenic effects. By using the tet-regulated system we have produced conditional transgenic mice overexpressing GSK-3beta in the brain during adulthood while avoiding perinatal lethality due to embryonic transgene expression. These mice show decreased levels of nuclear beta-catenin and hyperphosphorylation of tau in hippocampal neurons, the latter resulting in pretangle-like somatodendritic localization of tau. Neurons displaying somatodendritic localization of tau often show abnormal morphologies and detachment from the surrounding neuropil. Reactive astrocytosis and microgliosis were also indicative of neuronal stress and death. This was further confirmed by TUNEL and cleaved caspase-3 immunostaining of dentate gyrus granule cells. Our results demonstrate that in vivo overexpression of GSK-3beta results in neurodegeneration and suggest that these mice can be used as an animal model to study the relevance of GSK-3beta deregulation to the pathogenesis of Alzheimer's disease.
Figures
Fig. 1. Mouse design. (A) Schematic representation of the BitetO construct. This consists of seven copies of the palindromic tet operator sequence flanked by two CMV promoter sequences in divergent orientations. This bi-directional promoter is followed by a GSK-3β cDNA sequence (encoding a myc epitope at its 5′-end) in one direction and β-galactosidase (LacZ) sequence including a nuclear localization signal (NLS) in the other. (B) COS cells were co-transfected with the plasmid containing the BitetO construct and an expression vector coding for human tau (lanes 1–6), a third plasmid that allows expression of tTA was added (lanes 3–6) either in the absence (lanes 3 and 4) or in the presence (lanes 5 and 6) of 1 µg/ml tetracycline in the culture medium. Protein extracts were probed with antibodies against GSK-3β, AD-like phosphorylated tau (PHF-1), total tau (7.51) and β-galactosidase (β-Gal). (C) Tet/GSK-3β mice are generated by crossing mice expressing tTA under control of the CamKIIα promoter (tTA) with mice that have incorporated the BitetO construct in their genome (TetO). The double transgenic progeny (Tet/GSK-3β) are expected to express GSK-3β constitutively in the brain unless tetracycline or analogs are given orally thus preventing transactivation by tTA.
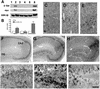
Fig. 2. Pattern of transgene expression in Tet/GSK-3β mice. (A and B) X-gal staining of brain sagittal sections from P0 Tet/GSK-3β mice that were either drug-naive (A) or born after giving doxycycline to the mother for 5 days immediately prior to birth (B). (C–E) β-galactosidase immunohistochemistry in sagittal sections of adult (3 months) Tet/GSK-3β mouse brain reveals expression in the different neuronal layers of the cortex (C), the hippocampus (D) and the striatum (E). H, hippocampus; Cx, cortex; St, striatum; II–VI, cortical layers; cc, corpus callosum; DG, dentate gyrus; Hil, hillus; GP, globus pallidus. Scale bar in (B) corresponds to 1 mm in (A) and (B). Scale bar in (E) corresponds to 200 µm in (C–E).
Fig. 3. Overexpression of GSK-3β in the cortex and hippocampus of Tet/GSK-3β mice. (A) Western blot of protein extracts from cortex (lanes 1 and 2), cerebellum (lanes 3 and 4), and hippocampus (lanes 5 and 6) of wild-type (lanes 1, 3 and 5) or Tet/GSK-3β (lanes 2, 4 and 6) mice. (B) Histogram showing percent increase of GSK-3β levels in Tet/GSK-3β mice. (C–E) Immunohistochemistry in cortical sections of wild-type (C) or Tet/GSK-3β (D and E) mice; performed with antibodies against GSK-3β (C and D) or myc (E). (F–H) Immunohistochemistry in hippocampal sections of wild-type (F) or Tet/GSK-3β (G and H) mice; performed with an antibody against GSK-3β (F and G) or myc (H). (I–K) High power magnification of the dentate gyri shown in (F–H). Scale bar corresponds to 100 µm in (C–E), 200 µm in (F–H), 60 µm in (I) and (J), and 40 µm in (K).
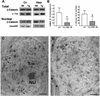
Fig. 4. Effect of GSK-3β overexpression on β-catenin levels. (A) Representative western blot of total cellular and nuclear preparations from the cortex (Cx) and hippocampus (Hipp) of wild-type (Wt) or Tet/GSK-3β (Tg) mice probed with anti-β-catenin antibody. β-tubulin and U2snRNP blots are shown as controls of total and nuclear protein loading. (B) Quantification of patches of β-catenin in the nucleus of dentate gyrus granule cells from wild-type and Tet/GSK-3β mice as detected by immunoelectron microscopy. (C) Electron microscopy photomicrograph showing a dentate gyrus neuron from a wild-type mouse with several patches of β-catenin reaction product (arrowheads). NU, nucleolus. (D) Electron microscopy photomicrograph showing a dentate gyrus neuron from a Tet/GSK-3β mouse. Asterisks, astrocitic process. No heavy metal staining was performed. Scale bars in (C) and (D) correspond to 0.1 µm.
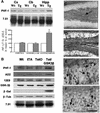
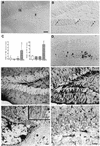
Fig. 5. Hyperphosphorylation and somatodendritic localization of tau in Tet/GSK-3β mice. (A) Western blot of protein extracts from cortex (Cx), cerebellum (Cb) and hippocampus (Hipp) of wild-type (Wt) or Tet/GSK-3β (Tg) mice probed with PHF-1 and 7.51 antibodies. Histogram shows the percentage of PHF-1/total (as demonstrated with 7.51 antibody) tau in Tet/GSK-3β mice with respect to wild-type mice. Note the significant increase in hippocampus (p < 0.05). (B) Western blot of hippocampal extracts from wild-type (Wt), tTA, TetO or Tet/GSK-3β mice probed with the indicated antibodies. (C–E) PHF-1 immunohistochemistry in the dentate gyrus of wild-type (C) or Tet/GSK-3β (D and E) mice. (F) Immunohistochemistry performed with 7.51 antibody in the dentate gyrus of a Tet/GSK-3β mouse. The arrow in (C) indicates faintly stained mossy fibers. The scale bar corresponds to 200 µm in (C) and (D), and 60 µm in (E) and (F).
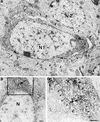
Fig. 6. Electron microscopy study of PHF-1 positive neurons. (A) Electron micrograph of two neurons from the dentate gyrus of Tet/GSK-3β mouse hippocampus. N1, nucleus of a PHF-1 immunopositive neuron exhibiting most of its perimeter detached from the surrounding neuropil (arrowheads), in addition to diffuse cytoplasmic immunostaining. N2, nucleus of a PHF-1 immunonegative neuron. (B) Another PHF-1 immunopositive neuron from the dentate gyrus of the same mouse as in (A), showing PHF-1 reaction product in patches and associated with RER. N, unlabeled nucleus. (C) high magnification of the framed portion in (B), showing the patches of reaction product (arrows) and the labeling of the RER (arrowheads). No heavy metal staining was performed. Calibration bar corresponds to 0.5 µm in all panels.
Fig. 7. Neuronal death and reactive gliosis in Tet/GSK-3β mice. (A and B) TUNEL staining of the dentate gyrus of wild-type (A) or Tet/GSK-3β (B) mice. Arrows indicate TUNEL positive nuclei. (C) Histograms showing the number of TUNEL positive neurons and of GFAP IR cells per 30 µm sagittal section of the dentate gyrus from mice of the four genotypes: wild-type, tTA, Tet-O and Tet/GSK-3β. Note the significant increases in Tet/GSK-3β mice with respect to wild type (*, p < 0.0005). (D) Immunohistochemistry against cleaved caspase-3 in the dentate gyrus of a Tet/GSK-3β mouse. (E and F) GFAP immunohistochemistry in the dentate gyrus of wild-type (E) or Tet/GSK-3β (F) mice. (G) Electron micrograph of the dentate gyrus of Tet/GSK-3β mouse hippocampus showing a hypertrophied astrocytic process (black star) adjacent to a PHF-1 immunostained neuron. N, nucleus. Asterisk, diffuse cytoplasmic PHF-1 immunostaining. Inset, high magnification of the astrocytic process showing characteristic bundles of glial intermediate filaments. (H) OX-42 immunostaining of the dentate gyrus of a Tet/GSK-3β mouse. Arrows indicate fine immunoreactive microglial processes. Arrowheads indicate immunostained reactive cell bodies. SM, stratum moleculare; SG, stratum granulare; H, hillus. Scale bars correspond to 50 µm in (A), (B) and (D); 60 µm in (E) and (F); 0.5 µm in (G); and 30 µm in (H).
Similar articles
- The active form of glycogen synthase kinase-3beta is associated with granulovacuolar degeneration in neurons in Alzheimer's disease.
Leroy K, Boutajangout A, Authelet M, Woodgett JR, Anderton BH, Brion JP. Leroy K, et al. Acta Neuropathol. 2002 Feb;103(2):91-9. doi: 10.1007/s004010100435. Epub 2001 Nov 29. Acta Neuropathol. 2002. PMID: 11810173 - GSK-3beta-dependent phosphorylation of adenomatous polyposis coli gene product can be modulated by beta-catenin and protein phosphatase 2A complexed with Axin.
Ikeda S, Kishida M, Matsuura Y, Usui H, Kikuchi A. Ikeda S, et al. Oncogene. 2000 Jan 27;19(4):537-45. doi: 10.1038/sj.onc.1203359. Oncogene. 2000. PMID: 10698523 - Change in tau phosphorylation associated with neurodegeneration in the ME7 model of prion disease.
Asuni AA, Perry VH, O'Connor V. Asuni AA, et al. Biochem Soc Trans. 2010 Apr;38(2):545-51. doi: 10.1042/BST0380545. Biochem Soc Trans. 2010. PMID: 20298219 Review. - Tau phosphorylation in hippocampus results in toxic gain-of-function.
Avila J, Gómez de Barreda E, Engel T, Lucas JJ, Hernández F. Avila J, et al. Biochem Soc Trans. 2010 Aug;38(4):977-80. doi: 10.1042/BST0380977. Biochem Soc Trans. 2010. PMID: 20658988 Review.
Cited by
- GSK-3 Mouse Models to Study Neuronal Apoptosis and Neurodegeneration.
Gómez-Sintes R, Hernández F, Lucas JJ, Avila J. Gómez-Sintes R, et al. Front Mol Neurosci. 2011 Nov 16;4:45. doi: 10.3389/fnmol.2011.00045. eCollection 2011. Front Mol Neurosci. 2011. PMID: 22110426 Free PMC article. - Diaminothiazoles modify Tau phosphorylation and improve the tauopathy in mouse models.
Zhang X, Hernandez I, Rei D, Mair W, Laha JK, Cornwell ME, Cuny GD, Tsai LH, Steen JA, Kosik KS. Zhang X, et al. J Biol Chem. 2013 Jul 26;288(30):22042-56. doi: 10.1074/jbc.M112.436402. Epub 2013 Jun 4. J Biol Chem. 2013. PMID: 23737518 Free PMC article. - Simple model systems reveal conserved mechanisms of Alzheimer's disease and related tauopathies.
Jiang Y, MacNeil LT. Jiang Y, et al. Mol Neurodegener. 2023 Nov 10;18(1):82. doi: 10.1186/s13024-023-00664-x. Mol Neurodegener. 2023. PMID: 37950311 Free PMC article. Review. - Alcohol Use Disorder, Neurodegeneration, Alzheimer's and Parkinson's Disease: Interplay Between Oxidative Stress, Neuroimmune Response and Excitotoxicity.
Kamal H, Tan GC, Ibrahim SF, Shaikh MF, Mohamed IN, Mohamed RMP, Hamid AA, Ugusman A, Kumar J. Kamal H, et al. Front Cell Neurosci. 2020 Aug 31;14:282. doi: 10.3389/fncel.2020.00282. eCollection 2020. Front Cell Neurosci. 2020. PMID: 33061892 Free PMC article. Review. - Discovery of a Highly Selective Glycogen Synthase Kinase-3 Inhibitor (PF-04802367) That Modulates Tau Phosphorylation in the Brain: Translation for PET Neuroimaging.
Liang SH, Chen JM, Normandin MD, Chang JS, Chang GC, Taylor CK, Trapa P, Plummer MS, Para KS, Conn EL, Lopresti-Morrow L, Lanyon LF, Cook JM, Richter KE, Nolan CE, Schachter JB, Janat F, Che Y, Shanmugasundaram V, Lefker BA, Enerson BE, Livni E, Wang L, Guehl NJ, Patnaik D, Wagner FF, Perlis R, Holson EB, Haggarty SJ, El Fakhri G, Kurumbail RG, Vasdev N. Liang SH, et al. Angew Chem Int Ed Engl. 2016 Aug 8;55(33):9601-5. doi: 10.1002/anie.201603797. Epub 2016 Jun 29. Angew Chem Int Ed Engl. 2016. PMID: 27355874 Free PMC article.
References
- Alvarez G., Munoz-Montano,J.R., Satrustegui,J., Avila,J., Bogonez,E. and Diaz-Nido,J. (1999) Lithium protects cultured neurons against β-amyloid-induced neurodegeneration. FEBS Lett., 453, 260–264. - PubMed
- Alzheimer A. (1911) Über eigernartige krankhertsfülle des späteren alters. Z. Gesamte Neurol. Psychiat., 4, 356–385.
- Anderton B.H. (1999) Alzheimer’s disease: clues from flies and worms. Curr. Biol., 9, R106–R109. - PubMed
- Barth A.I., Nathke,I.S. and Nelson,W.J. (1997) Cadherins, catenins and APC protein: interplay between cytoskeletal complexes and signaling pathways. Curr. Opin. Cell Biol., 9, 683–690. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous