Supernatant protein factor, which stimulates the conversion of squalene to lanosterol, is a cytosolic squalene transfer protein and enhances cholesterol biosynthesis - PubMed (original) (raw)
Supernatant protein factor, which stimulates the conversion of squalene to lanosterol, is a cytosolic squalene transfer protein and enhances cholesterol biosynthesis
N Shibata et al. Proc Natl Acad Sci U S A. 2001.
Free PMC article
Abstract
Squalene epoxidase, a membrane-associated enzyme that converts squalene to squalene 2,3-oxide, plays an important role in the maintenance of cholesterol homeostasis. In 1957, Bloch and colleagues identified a factor from rat liver cytosol termed "supernatant protein factor (SPF)," which promotes the squalene epoxidation catalyzed by rat liver microsomes with oxygen, NADPH, FAD, and phospholipid [Tchen, T. T. & Bloch, K. (1957) J. Biol. Chem. 226, 921-930]. Although purification of SPF by 11,000-fold was reported, no information is so far available on the primary structure or biological function of SPF. Here we report the cDNA cloning and expression of SPF from rat and human. The encoded protein of 403 amino acids belongs to a family of cytosolic lipid-binding/transfer proteins such as alpha-tocopherol transfer protein, cellular retinal binding protein, yeast phosphatidylinositol transfer protein (Sec14p), and squid retinal binding protein. Recombinant SPF produced in Escherichia coli enhances microsomal squalene epoxidase activity and promotes intermembrane transfer of squalene in vitro. SPF mRNA is expressed abundantly in the liver and small intestine, both of which are important sites of cholesterol biosynthesis. SPF is expressed significantly in isolated hepatocytes, but the expression level was markedly decreased after 48 h of in vitro culture. Moreover, SPF was not detectable in most of the cell lines tested, including HepG2 and McARH7777 hepatomas. Transfection of SPF cDNA in McARH7777 significantly stimulated de novo cholesterol biosynthesis. These data suggest that SPF is a cytosolic squalene transfer protein capable of regulating cholesterol biosynthesis.
Figures
Figure 1
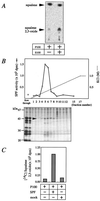
Purification of SPF from rat liver. (A) In vitro SE-promoting activity of rat liver cytosol. [14C]Squalene and rat liver microsome (P100) were incubated at 37°C for 30 min in the presence or absence of rat liver cytosol (S100), and the production of [14C]squalene 2,3-oxide was examined by TLC and bioimage analyzer. (B) Elution profile of SPF activities from a Mono S HR5/5 column at pH 6.5. Fractions were subjected to SDS/PAGE and silver staining. The arrowhead indicates the 45-kDa band that paralleled SPF activities. (C) SE-promoting activities of recombinant SPF. McARH7777 cells were plated on day 0 at a density of 1.0 × 106 cells per square centimeter into 100-mm collagen-coated dishes. On day 1, transfection of pcDNA3-SPF or pcDNA3 (mock) was performed. On day 3, cells were harvested and homogenized in SET buffer, and the cytosol was used for the SPF activity assay.
Figure 2
Primary structure of SPF. (A) Predicted amino acid sequences of rat and human SPFs. Identical residues are highlighted in black. Sequence for human SPF (GenBank accession no. AL096881) was obtained from the
blast
database. GenBank accession no. for rat SPF is AF309558. (B) Comparison of SPF sequence with other homologous proteins. SPF-like protein sequence is from rat (GenBank accession no. AJ132352). Retinal binding protein is from squid (GenBank accession no. S68871). Sec14p is from Saccharomyces cerevisiae (European Molecular Biology Laboratory accession no. Z49259). α-TTP is from rat (GenBank accession no. D49488). Cellular retinal binding protein is from human (GenBank accession no. L34219). Indicated values shown above are pairwise identities in relation to rat SPF within each domain. Sequences within the highly conserved domains (i and ii) are shown (Bottom). Residues that are identical in at least three of the aligned sequences are highlighted in black. Similar residues are shaded in gray. Abbreviations for the amino acid residues are as follows: A, Ala; C, Cys; D, Asp; E, Glu; F, Phe; G, Gly; H, His; I, Ile; K, Lys; L, Leu; M, Met; N, Asn; P, Pro; Q, Gln; R, Arg; S, Ser; T, Thr; V, Val; W, Trp; Y, Tyr.
Figure 3
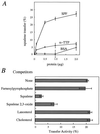
Recombinant SPF stimulates squalene transfer. (A) Dose dependence of squalene transfer. Egg phosphatidylcholine liposomes containing [3H]squalene were incubated with the indicated concentration of rat SPF (○), rat α-TTP (□), or BSA (▵) and rat liver heavy membrane for 30 min at 37°C, and the transfer of [3H]squalene was determined. (B) Effect of the incorporation of unlabeled substances into liposomes on the transfer of [3H]squalene. Liposomes containing [3H]squalene (5.5 × 104 dpm) and a 100-fold excess of unlabeled substances were incubated with rat liver heavy membrane in the presence of 1.0 μg of recombinant SPF for 30 min at 37°C. Each point denotes the mean ± SE of three separate experiments.
Figure 4
Tissue distribution of rat SPF mRNA. Poly (A)+ RNA (2 μg) from various rat tissues was analyzed with a random-primed32P-labeled rat SPF cDNA probe.
Figure 5
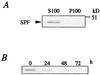
Western blot analysis of SPF. (A) Immunoblot analysis of S100 and P100 fractions from rat liver with anti-rat SPF polyclonal antibody. (B) Expression of SPF in rat hepatocyte primary culture. The harvested cells were subjected to immunoblotting with anti-rat SPF polyclonal antibody.
Figure 6
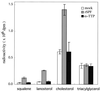
Effect of expression of SPF on the incorporation of [14C]acetate into cellular sterols in McARH7777 cells. McARH7777 cells were transiently transfected by cDNA plasmids [mock (□), rat SPF (░⃞), rat α-TTP (■)], and after 48 h, [14C]acetate was added to a final concentration of 28 μCi/ml, and the cells were incubated for 3 h. Radiolabeled sterols were extracted and resolved by TLC, and the incorporated radioactivities of squalene, lanosterol, cholesterol, and triacylglycerol were measured. Each point denotes the mean ± SE of three separate experiments.
Similar articles
- Ligand specificity in the CRAL-TRIO protein family.
Panagabko C, Morley S, Hernandez M, Cassolato P, Gordon H, Parsons R, Manor D, Atkinson J. Panagabko C, et al. Biochemistry. 2003 Jun 3;42(21):6467-74. doi: 10.1021/bi034086v. Biochemistry. 2003. PMID: 12767229 - Supernatant protein factor stimulates HMG-CoA reductase in cell culture and in vitro.
Mokashi V, Singh DK, Porter TD. Mokashi V, et al. Arch Biochem Biophys. 2005 Jan 15;433(2):474-80. doi: 10.1016/j.abb.2004.10.002. Arch Biochem Biophys. 2005. PMID: 15581604 - Molecular mechanisms of vitamin E transport.
Stocker A. Stocker A. Ann N Y Acad Sci. 2004 Dec;1031:44-59. doi: 10.1196/annals.1331.005. Ann N Y Acad Sci. 2004. PMID: 15753133 Review. - Supernatant protein factor and tocopherol-associated protein: an unexpected link between cholesterol synthesis and vitamin E (review).
Porter TD. Porter TD. J Nutr Biochem. 2003 Jan;14(1):3-6. doi: 10.1016/s0955-2863(02)00262-0. J Nutr Biochem. 2003. PMID: 12559471 Review. - Effects of a supernatant protein activator on microsomal squalene-2,3-oxide-lanosterol cyclase.
Caras IW, Bloch K. Caras IW, et al. J Biol Chem. 1979 Dec 10;254(23):11816-21. J Biol Chem. 1979. PMID: 500676
Cited by
- Demonstrated and inferred metabolism associated with cytosolic lipid droplets.
Goodman JM. Goodman JM. J Lipid Res. 2009 Nov;50(11):2148-56. doi: 10.1194/jlr.R001446. Epub 2009 Aug 20. J Lipid Res. 2009. PMID: 19696439 Free PMC article. Review. - Expression of tocopherol-associated protein in mast cells.
Ikeda T, Murakami M, Funaba M. Ikeda T, et al. Clin Diagn Lab Immunol. 2004 Nov;11(6):1189-91. doi: 10.1128/CDLI.11.6.1189-1191.2004. Clin Diagn Lab Immunol. 2004. PMID: 15539527 Free PMC article. - Metabolite trafficking enables membrane-impermeable-terpene secretion by yeast.
Son SH, Kim JE, Park G, Ko YJ, Sung BH, Seo J, Oh SS, Lee JY. Son SH, et al. Nat Commun. 2022 May 11;13(1):2605. doi: 10.1038/s41467-022-30312-9. Nat Commun. 2022. PMID: 35546160 Free PMC article. - Role of SEC14-like phosphatidylinositol transfer proteins in membrane identity and dynamics.
Montag K, Ivanov R, Bauer P. Montag K, et al. Front Plant Sci. 2023 May 15;14:1181031. doi: 10.3389/fpls.2023.1181031. eCollection 2023. Front Plant Sci. 2023. PMID: 37255567 Free PMC article. Review. - Mechanisms for the prevention of vitamin E excess.
Traber MG. Traber MG. J Lipid Res. 2013 Sep;54(9):2295-306. doi: 10.1194/jlr.R032946. Epub 2013 Mar 15. J Lipid Res. 2013. PMID: 23505319 Free PMC article. Review.
References
- Tchen T T, Bloch K. J Biol Chem. 1957;226:921–930. - PubMed
- Yamamoto S, Bloch K. J Biol Chem. 1970;245:1670–1674. - PubMed
- Tai H-H, Bloch K. J Biol Chem. 1972;247:3767–3773. - PubMed
- Ono T, Bloch K. J Biol Chem. 1975;250:1571–1579. - PubMed
- Ferguson J B, Bloch K. J Biol Chem. 1977;252:5381–5385. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases