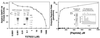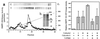Identification of the endogenous smooth muscle myosin phosphatase-associated kinase - PubMed (original) (raw)
Identification of the endogenous smooth muscle myosin phosphatase-associated kinase
J A MacDonald et al. Proc Natl Acad Sci U S A. 2001.
Abstract
Ca(2+) sensitization of smooth muscle contraction involves inhibition of myosin light chain phosphatase (SMPP-1M) and enhanced myosin light chain phosphorylation. Inhibition of SMPP-1M is modulated through phosphorylation of the myosin targeting subunit (MYPT1) by either Rho-associated kinase (ROK) or an unknown SMPP-1M-associated kinase. Activated ROK is predominantly membrane-associated and its putative substrate, SMPP-1M, is mainly myofibrillar-associated. This raises a conundrum about the mechanism of interaction between these enzymes. We present ZIP-like kinase, identified by "mixed-peptide" Edman sequencing after affinity purification, as the previously unidentified SMPP-1M-associated kinase. ZIP-like kinase was shown to associate with MYPT1 and phosphorylate the inhibitory site in intact smooth muscle. Phosphorylation of ZIP-like kinase was associated with an increase in kinase activity during carbachol stimulation, suggesting that the enzyme may be a terminal member of a Ca(2+) sensitizing kinase cascade.
Figures
Figure 1
Determination of the sites of phosphorylation of MYPT1 in vivo. (A) [32P]orthophosphate-labeled rabbit bladder was stimulated with 10 μM carbachol for the indicated times. MYPT1 was immunoprecipitated from tissue homogenates then resolved by SDS/PAGE. Increased MYPT1 phosphorylation was determined by autoradiography. (B) MYPT1 immunoprecipitated from control and carbachol stimulated rabbit bladder was digested overnight with trypsin; the32P-labeled peptides obtained were separated on a C18 reverse-phase column and identified by scintillation counting. (Inset) One of the phosphorylated MYPT1 peptides (no. 2) was sequenced and its phosphorylation site identified as described (32).
Figure 2
Endogenous kinase copurifies with SMPP-1M. (Inset in A) Autoradiography of purified SMPP-1M shows a phosphorylated band at 110 kDa, correlating with MYPT1. SMPP-1M was affinity purified as described (13) and the purified enzyme incubated with 100 μM [γ-32P]ATP and 2 mM MgCl2. The reaction was terminated with sample buffer and MYPT1 resolved on SDS/PAGE gels. (B) Purified M110 kinases accelerates the rate of SMPP-1M inactivation in vitro. Purified SMPP-1M was incubated for the indicated times with Mg/ATP (2 mM/100 μM) in the presence (○) or absence (●) of affinity purified M110 kinase. Note: Inactivation of SMPP-1M in the absence of exogenously added M110 kinase was due to the presence of endogenous copurifying kinase activity. (A) The myofibrillar extract from rabbit bladder was resolved on an AP-1Q (0.5 cm × 7 cm) anion exchange column; the column was developed with a 0–1-M NaCl gradient. SMPP-1M (●) was assayed against32P-labeled myosin and SMPP-1M kinase activity (λ) was assayed against the Thr697 substrate peptide (KKKRQSRRSTQGVTL).
Figure 3
Purification of SMPP-1M-associated kinase. (A) SMPP-1M kinase was eluted from a SMART MiniQ (1.6/5) anion exchange column with a 0–1-M NaCl gradient and identified by using both in vitro and in-gel kinase assays. (Inset b) The autoradiogram of the in-gel assay localized kinase activity to a discrete protein band at 32 kDa. (Inset c) The results obtained from phosphoamino acid analysis (33) of Thr697 substrate peptide phosphorylated during the in vitro assay by purified SMPP-1M kinase. Phosphorylated Thr697 substrate peptide was sequenced and its phosphorylation site determined as described (32). (Inset d) In-gel kinase assay comparing purified PKA (1 μg) control with purified SMPP-1M kinase. A control gel run in the absence of Thr697 substrate peptide was blank.
Figure 4
Identification of SMPP-1M-associated kinase by mixed peptide sequencing. Mixed sequence is listed in order of the PTH amino acids recovered after each Edman cycle. Sequence data shown was derived from 200 fmol of protein.
fastf
was used to search and match the mixed sequences to the National Center for Biotechnology Information (NCBI)/Human protein database. The scoring matrix was MD20, with expectation and score values set to <1 and 5, respectively (23). The highest scoring proteins were: human ZIPK, (e) 5.1 e-14; human pDAPK3, (e) 5.1 e-14; and rat DAP-like kinase, (e) 2.1 e-7. The next highest unrelated protein score was
d
-glycerate dehydrogenase, (e) 0.0011.
Figure 5
ZIP-like kinase properties toward MYPT1. (A) Effect of ROK inhibitor Y-27632 on ZIPK and ZIP-like kinase. Kinases were assayed_in vitro_ against the Thr697 peptide. (B) Substrate concentration dependence of purified bladder ZIP kinase (○), and ROK (●). (Inset C) Autoradiograms showing phosphorylation of chicken gizzard full-length MYPT1 (18), rM133, and chicken gizzard C-terminal fragment (C130514–963; ref. 34) by purified bladder ZIPK and ROK in vitro. Data are means ± SEM of three separate experiments. (Inset D) Identification of the autophosphorylation sites on ZIPK.
Figure 6
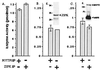
Association of SMPP-1M with ZIP-like kinase. (A) MYPT1 was immunoprecipitated and ZIP-like kinase measured in the immunoprecipitate. Alternatively, ZIP-like kinase was immunoprecipitated and myosin phosphatase measured against (B) glycogen phosphorylase a or (C) myosin. (Inset d) Tissue extracts from bladder were immunoprecipitated with anti-MYPT1 antibody, resolved on SDS/PAGE, and immunoblotted for ZIPK. (Inset e) Tissue extracts from bladder were immunoprecipitated with anti-ZIPK antibody, resolved on SDS/PAGE, and immunoblotted for MYPT1.
Figure 7
Carbachol affects ZIP-like kinase phosphorylation and activity in smooth muscle. [32P]orthophosphate-labeled rabbit bladder was stimulated with 50 μM carbachol in the presence of 10 μM calyculin A. Triton-extracted tissue pellets were fractionated on a SMART MiniQ (1.6/5 cm) column. (a) Aliquots of fractions were run on SDS/PAGE gels and subjected to autoradiography (b) to visualize phosphorylation. Western immunoblots were used to identify the protein bands that corresponded with ZIPK. SMART fractions from both control (C) and carbachol-treated (T) bladder containing ZIP-like kinase were pooled, immunoprecipitated with anti-ZIP kinase antibody, and resolved on SDS/PAGE before autoradiography (b). (b) Carbachol/calyculin A treatment increase ZIP-like kinase activity. Homogenates were prepared and MYPT1 was immunoprecipitated. Immunoprecipitates were assessed in duplicate for ZIP-like kinase activity. Activity shown was derived following subtraction of nonspecific background kinase activity that was also present in the immunoprecipitate. Data represent the means ± SEM of five separate experiments. *, significantly different from the control value by the Student-Newman-Keuls test, P < 0.05; **, significantly different from the carbachol/calyculin A treatment, P < 0.05.
Similar articles
- Identification and characterization of zipper-interacting protein kinase as the unique vascular smooth muscle myosin phosphatase-associated kinase.
Endo A, Surks HK, Mochizuki S, Mochizuki N, Mendelsohn ME. Endo A, et al. J Biol Chem. 2004 Oct 1;279(40):42055-61. doi: 10.1074/jbc.M403676200. Epub 2004 Jul 30. J Biol Chem. 2004. PMID: 15292222 - Smooth muscle phosphatase is regulated in vivo by exclusion of phosphorylation of threonine 696 of MYPT1 by phosphorylation of Serine 695 in response to cyclic nucleotides.
Wooldridge AA, MacDonald JA, Erdodi F, Ma C, Borman MA, Hartshorne DJ, Haystead TA. Wooldridge AA, et al. J Biol Chem. 2004 Aug 13;279(33):34496-504. doi: 10.1074/jbc.M405957200. Epub 2004 Jun 11. J Biol Chem. 2004. PMID: 15194681 - Smooth muscle myosin phosphatase-associated kinase induces Ca2+ sensitization via myosin phosphatase inhibition.
Borman MA, MacDonald JA, Murányi A, Hartshorne DJ, Haystead TA. Borman MA, et al. J Biol Chem. 2002 Jun 28;277(26):23441-6. doi: 10.1074/jbc.M201597200. Epub 2002 Apr 25. J Biol Chem. 2002. PMID: 11976330 - ZIP kinase, a key regulator of myosin protein phosphatase 1.
Haystead TA. Haystead TA. Cell Signal. 2005 Nov;17(11):1313-22. doi: 10.1016/j.cellsig.2005.05.008. Cell Signal. 2005. PMID: 16005610 Review. - The regulation of smooth muscle contractility by zipper-interacting protein kinase.
Ihara E, MacDonald JA. Ihara E, et al. Can J Physiol Pharmacol. 2007 Jan;85(1):79-87. doi: 10.1139/y06-103. Can J Physiol Pharmacol. 2007. PMID: 17487247 Review.
Cited by
- The p90 ribosomal S6 kinase (RSK) is a mediator of smooth muscle contractility.
Artamonov M, Momotani K, Utepbergenov D, Franke A, Khromov A, Derewenda ZS, Somlyo AV. Artamonov M, et al. PLoS One. 2013;8(3):e58703. doi: 10.1371/journal.pone.0058703. Epub 2013 Mar 13. PLoS One. 2013. PMID: 23516539 Free PMC article. - Physiology of endothelin and the kidney.
Kohan DE, Inscho EW, Wesson D, Pollock DM. Kohan DE, et al. Compr Physiol. 2011 Apr;1(2):883-919. doi: 10.1002/cphy.c100039. Compr Physiol. 2011. PMID: 23737206 Free PMC article. Review. - Myosin phosphatase is inactivated by caspase-3 cleavage and phosphorylation of myosin phosphatase targeting subunit 1 during apoptosis.
Iwasaki T, Katayama T, Kohama K, Endo Y, Sawasaki T. Iwasaki T, et al. Mol Biol Cell. 2013 Mar;24(6):748-56. doi: 10.1091/mbc.E11-08-0740. Epub 2013 Jan 23. Mol Biol Cell. 2013. PMID: 23345589 Free PMC article. - Molecular mechanism of telokin-mediated disinhibition of myosin light chain phosphatase and cAMP/cGMP-induced relaxation of gastrointestinal smooth muscle.
Khromov AS, Momotani K, Jin L, Artamonov MV, Shannon J, Eto M, Somlyo AV. Khromov AS, et al. J Biol Chem. 2012 Jun 15;287(25):20975-85. doi: 10.1074/jbc.M112.341479. Epub 2012 Apr 27. J Biol Chem. 2012. PMID: 22544752 Free PMC article. - Integrin-linked kinase phosphorylates the myosin phosphatase target subunit at the inhibitory site in platelet cytoskeleton.
Kiss E, Murányi A, Csortos C, Gergely P, Ito M, Hartshorne DJ, Erdodi F. Kiss E, et al. Biochem J. 2002 Jul 1;365(Pt 1):79-87. doi: 10.1042/BJ20011295. Biochem J. 2002. PMID: 11931630 Free PMC article.
References
- Hartshorne D J. In: Physiology of the Gastrointestinal Tract. Johnson L R, editor. New York: Raven; 1987. pp. 423–482.
- Sellers J R, Adelstein R S. Curr Top Cell Regul. 1985;27:51–62. - PubMed
- Somlyo A P, Somlyo A V. In: The Heart and Cardiovascular System. Fozzard H A, editor. New York: Raven; 1991. pp. 1–30.
- Somlyo A P, Somlyo A V. Nature (London) 1994;372:231–236. - PubMed
- Hartshorne D J, Ito M, Erdodi F. J Muscle Res Cell Motil. 1998;19:325–341. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL23615/HL/NHLBI NIH HHS/United States
- DK52378-04/DK/NIDDK NIH HHS/United States
- P01 HL019242/HL/NHLBI NIH HHS/United States
- P01 HL19242-24/HL/NHLBI NIH HHS/United States
- R01 HL023615/HL/NHLBI NIH HHS/United States
- R01 DK052378/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous