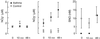NO chemical events in the human airway during the immediate and late antigen-induced asthmatic response - PubMed (original) (raw)
NO chemical events in the human airway during the immediate and late antigen-induced asthmatic response
R A Dweik et al. Proc Natl Acad Sci U S A. 2001.
Abstract
A wealth of evidence supports increased NO (NO.) in asthma, but its roles are unknown. To investigate how NO participates in inflammatory airway events in asthma, we measured NO. and NO. chemical reaction products [nitrite, nitrate, S-nitrosothiols (SNO), and nitrotyrosine] before, immediately and 48 h after bronchoscopic antigen (Ag) challenge of the peripheral airways in atopic asthmatic individuals and nonatopic healthy controls. Strikingly, NO(3)(-) was the only NO. derivative to increase during the immediate Ag-induced asthmatic response and continued to increase over 2-fold at 48 h after Ag challenge in contrast to controls [P < 0.05]. NO(2)(-) was not affected by Ag challenge at 10 min or 48 h after Ag challenge. Although SNO was not detectable in asthmatic airways at baseline or immediately after Ag, SNO increased during the late response to levels found in healthy controls. A model of NO. dynamics derived from the current findings predicts that NO. may have harmful effects through formation of peroxynitrite, but also subserves an antioxidant role by consuming reactive oxygen species during the immediate asthmatic response, whereas nitrosylation during the late asthmatic response generates SNO, safe reservoirs for removal of toxic NO. derivatives.
Figures
Figure 1
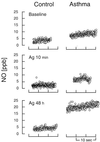
An asthmatic individual with higher NO⋅ plateau in intrapulmonary gases than a healthy control individual at Baseline before Ag challenge. At 10 min after Ag challenge (Ag 10 min), NO⋅ plateau levels are similar to baseline levels, but by 48 h after Ag challenge (Ag 48 h), the NO⋅ plateau in the asthmatic airway gases is much higher than plateau levels at baseline. In contrast, the nonatopic healthy control had no increase in plateau NO⋅ in intrapulmonary gases. Each point represents a single NO⋅ determination in gases continually sampled from the lower airway at bronchoscopy at intervals of 0.05 sec.
Figure 2
NO metabolites in BAL fluid from asthmatic and control airways before (0 min), and 10 min or 48 h after segmental Ag challenge. NO in asthmatic or control BAL fluid did not change significantly with Ag challenge from baseline (all comparisons.P > 0.5). NO
increases at 10 min and 48 h after Ag challenge in asthmatics, whereas NO
did not change in control airways. SNO were undetectable in the asthmatic airway at baseline and 10 min after Ag challenge but increased at 48 h after Ag challenge.
Figure 3
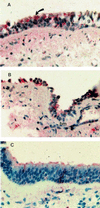
Nitrotyrosine immunostaining of asthmatic and healthy control bronchial mucosa. (A) Asthmatic bronchial mucosa has marked increase in goblet cells and thickened basement membrane. The epithelial cells stain prominently for nitrotyrosine (red staining); red, arrow; g, goblet cell. (B) At 48 h after Ag challenge, epithelial cells are sloughing from the thickened basement membrane (bm), but the cells remaining show positivity for nitrotyrosine. (C) Healthy control bronchial mucosa shows typical pseudostratified columnar epithelium, with red staining present in apical portions of cells (×40; hematoxylin counterstaining).
Figure 4
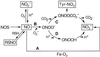
Model of NO⋅ reactions in the lung during an Ag-induced asthmatic response.
Similar articles
- Immediate and late inflammatory responses to ragweed antigen challenge of the peripheral airways in allergic asthmatics. Cellular, mediator, and permeability changes.
Liu MC, Hubbard WC, Proud D, Stealey BA, Galli SJ, Kagey-Sobotka A, Bleecker ER, Lichtenstein LM. Liu MC, et al. Am Rev Respir Dis. 1991 Jul;144(1):51-8. doi: 10.1164/ajrccm/144.1.51. Am Rev Respir Dis. 1991. PMID: 2064141 - Smoking attenuates increase in exhaled nitric oxide in atopic but not in nonatopic young adults with asthma.
Rouhos A, Ekroos H, Karjalainen J, Sarna S, Haahtela T, Sovijärvi AR. Rouhos A, et al. Int Arch Allergy Immunol. 2010;152(3):226-32. doi: 10.1159/000283029. Epub 2010 Feb 11. Int Arch Allergy Immunol. 2010. PMID: 20150740 - Nitric oxide metabolism in asthma pathophysiology.
Ghosh S, Erzurum SC. Ghosh S, et al. Biochim Biophys Acta. 2011 Nov;1810(11):1008-16. doi: 10.1016/j.bbagen.2011.06.009. Epub 2011 Jun 21. Biochim Biophys Acta. 2011. PMID: 21718755 Free PMC article. Review. - Nitric oxide, peroxynitrite, and lower respiratory tract inflammation.
Robbins RA, Hadeli K, Nelson D, Sato E, Hoyt JC. Robbins RA, et al. Immunopharmacology. 2000 Jul 25;48(3):217-21. doi: 10.1016/s0162-3109(00)00220-4. Immunopharmacology. 2000. PMID: 10960660 Review. No abstract available.
Cited by
- Benefits of Airway Androgen Receptor Expression in Human Asthma.
Zein JG, McManus JM, Sharifi N, Erzurum SC, Marozkina N, Lahm T, Giddings O, Davis MD, DeBoer MD, Comhair SA, Bazeley P, Kim HJ, Busse W, Calhoun W, Castro M, Chung KF, Fahy JV, Israel E, Jarjour NN, Levy BD, Mauger DT, Moore WC, Ortega VE, Peters M, Bleecker ER, Meyers DA, Zhao Y, Wenzel SE, Gaston B. Zein JG, et al. Am J Respir Crit Care Med. 2021 Aug 1;204(3):285-293. doi: 10.1164/rccm.202009-3720OC. Am J Respir Crit Care Med. 2021. PMID: 33779531 Free PMC article. - Proteomic method identifies proteins nitrated in vivo during inflammatory challenge.
Aulak KS, Miyagi M, Yan L, West KA, Massillon D, Crabb JW, Stuehr DJ. Aulak KS, et al. Proc Natl Acad Sci U S A. 2001 Oct 9;98(21):12056-61. doi: 10.1073/pnas.221269198. Epub 2001 Oct 2. Proc Natl Acad Sci U S A. 2001. PMID: 11593016 Free PMC article. - Myeloperoxidase up-regulates the catalytic activity of inducible nitric oxide synthase by preventing nitric oxide feedback inhibition.
Galijasevic S, Saed GM, Diamond MP, Abu-Soud HM. Galijasevic S, et al. Proc Natl Acad Sci U S A. 2003 Dec 9;100(25):14766-71. doi: 10.1073/pnas.2435008100. Epub 2003 Dec 1. Proc Natl Acad Sci U S A. 2003. PMID: 14657339 Free PMC article. - Detrimental effects of environmental tobacco smoke in relation to asthma severity.
Comhair SA, Gaston BM, Ricci KS, Hammel J, Dweik RA, Teague WG, Meyers D, Ampleford EJ, Bleecker ER, Busse WW, Calhoun WJ, Castro M, Chung KF, Curran-Everett D, Israel E, Jarjour WN, Moore W, Peters SP, Wenzel S, Hazen SL, Erzurum SC; National Heart Lung Blood Institute Severe Asthma Research Program. Comhair SA, et al. PLoS One. 2011 May 4;6(5):e18574. doi: 10.1371/journal.pone.0018574. PLoS One. 2011. PMID: 21572527 Free PMC article. - Redox control of asthma: molecular mechanisms and therapeutic opportunities.
Comhair SA, Erzurum SC. Comhair SA, et al. Antioxid Redox Signal. 2010 Jan;12(1):93-124. doi: 10.1089/ars.2008.2425. Antioxid Redox Signal. 2010. PMID: 19634987 Free PMC article. Review.
References
- Persson M G, Zetterstrom O, Agrenius V, Ihre E, Gustafsson L E. Lancet. 1994;343:146–147. - PubMed
- Kharitonov S A, Yates D, Robbins R A, Logan-Sinclair R, Shinebourne E A, Barnes P J. Lancet. 1994;343:133–135. - PubMed
- Massaro A, Mehta S, Lilly C, Kobzik L, Reilly J, Drazen J. Am J Respir Crit Care Med. 1996;153:1510–1514. - PubMed
- Guo F H, Comhair S A A, Zheng S, Dweik R A, Eissa N T, Thomassen M J, Calhoun W, Erzurum S C. J Immunol. 2000;164:5970–5980. - PubMed
- Silkoff P, Sylvester J, Zamel N, Permutt S. Am J Respir Crit Care Med. 2000;161:1218–1228. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical