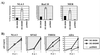DC-SIGNR, a DC-SIGN homologue expressed in endothelial cells, binds to human and simian immunodeficiency viruses and activates infection in trans - PubMed (original) (raw)
DC-SIGNR, a DC-SIGN homologue expressed in endothelial cells, binds to human and simian immunodeficiency viruses and activates infection in trans
S Pöhlmann et al. Proc Natl Acad Sci U S A. 2001.
Abstract
DC-SIGN, a C-type lectin expressed on the surface of dendritic cells (DCs), efficiently binds and transmits HIVs and simian immunodeficiency viruses to susceptible cells in trans. A DC-SIGN homologue, termed DC-SIGNR, has recently been described. Herein we show that DC-SIGNR, like DC-SIGN, can bind to multiple strains of HIV-1, HIV-2, and simian immunodeficiency virus and transmit these viruses to both T cell lines and human peripheral blood mononuclear cells. Binding of virus to DC-SIGNR was dependent on carbohydrate recognition. Immunostaining with a DC-SIGNR-specific antiserum showed that DC-SIGNR was expressed on sinusoidal endothelial cells in the liver and on endothelial cells in lymph node sinuses and placental villi. The presence of this efficient virus attachment factor on multiple endothelial cell types indicates that DC-SIGNR could play a role in the vertical transmission of primate lentiviruses, in the enabling of HIV to traverse the capillary endothelium in some organs, and in the presentation of virus to CD4-positive cells in multiple locations including lymph nodes.
Figures
Figure 1
DC-SIGNR specific antiserum. Human 293T cells transiently expressing AU1-tagged versions of DC-SIGN, DC-SIGNR, or pcDNA3 vector alone were lysed in nonionic detergent. Aliquots of the resulting lysates were analyzed by SDS/PAGE and Western blotting. Blots were probed with a mAb directed against the AU1-antigenic tag (A) or with an antiserum raised against a peptide based on a unique sequence in the cytoplasmic domain of DC-SIGNR (B).
Figure 2
Expression of DC-SIGNR. Formalin-fixed paraffin-embedded sections of human tissues were immunostained with anti-DC-SIGNR chicken serum or anti-Von Willebrand factor rabbit serum. (A and_C_) Normal lymph node: the endothelial cells lining the sinuses are stained strongly with anti-DC-SIGNR. (B and_D_) Normal lymph node: an identical pattern of staining with anti-Von Willebrand factor is observed in the lymph node sinusoids. Anti-Von Willebrand factor also stained high endothelial vessels that did not stain with anti-DC-SIGNR. (E) Villi from normal term placenta: the endothelium of approximately half of the capillaries is stained with anti-DC-SIGNR antibody. (F) Villi from normal term placenta: all capillaries are stained with anti-Von Willebrand factor. (G) Normal liver: anti-DC-SIGNR antibody stained all sinusoids. (H) Normal liver: anti-Von Willebrand factor confirmed the identity of the sinusoidal lining cells as endothelium.
Figure 3
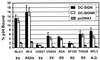
Binding of HIV to DC-SIGNR transfected cells. 293T cells were transiently transfected with the indicated expression plasmids. The cells were incubated with p24-normalized virus stocks, vigorously washed, and lysed in 0.5% Triton X-100, and then the content of bound p24 was quantified by ELISA. The tropism of each virus is indicated (R5, X4, or R5X4). All viruses are clade B except for the UG021 and UG024 isolates, which are clade D. Data are presented as the percent of recovered p24 antigen. The mean values ± SEM of four experiments are shown. N.D., not done.
Figure 4
DC-SIGNR mediates transmission of HIV-1, HIV-2, and SIV strains. DC-SIGNR, DC-SIGN, or pcDNA3 control plasmids were transiently transfected into 293T cells. The cells were pulsed with virus, extensively washed, and cocultivated with target cells. (A) Transmission of luciferase-reporter viruses to C8166 T cells. HIV-1 NL4–3, HIV-2 Rod10, and SIVmac239 MER Env luciferase reporter viruses were used in the transfer assay described above. Luciferase activity was determined 3 days after the start of the cocultivation. Data are the mean ± SEM of three experiments. (B) Transmission of HIV isolates to PBMCs. The transmission of primary HIV isolates and the laboratory-adapted NL4–3 virus was investigated as described above. The culture supernatants were removed as indicated (d.p.i., days postinfection), and their p24 content was measured by ELISA. Similar results were obtained in an independent experiment.
Figure 5
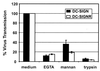
EGTA, mannan, and trypsin/EDTA inhibit virus transfer. The transfer of NL4–3 luciferase reporter virus was analyzed as described above, except that the 293T cells were preincubated with EGTA or with mannan or one of the three wash steps was carried out with trypsin/EDTA instead of medium.
Similar articles
- Expression of human immunodeficiency virus (HIV)-binding lectin DC-SIGNR: Consequences for HIV infection and immunity.
Soilleux EJ, Morris LS, Rushbrook S, Lee B, Coleman N. Soilleux EJ, et al. Hum Pathol. 2002 Jun;33(6):652-9. doi: 10.1053/hupa.2002.124036. Hum Pathol. 2002. PMID: 12152166 - DC-SIGN interactions with human immunodeficiency virus type 1 and 2 and simian immunodeficiency virus.
Pöhlmann S, Baribaud F, Lee B, Leslie GJ, Sanchez MD, Hiebenthal-Millow K, Münch J, Kirchhoff F, Doms RW. Pöhlmann S, et al. J Virol. 2001 May;75(10):4664-72. doi: 10.1128/JVI.75.10.4664-4672.2001. J Virol. 2001. PMID: 11312337 Free PMC article. - Quantitative expression and virus transmission analysis of DC-SIGN on monocyte-derived dendritic cells.
Baribaud F, Pöhlmann S, Leslie G, Mortari F, Doms RW. Baribaud F, et al. J Virol. 2002 Sep;76(18):9135-42. doi: 10.1128/jvi.76.18.9135-9142.2002. J Virol. 2002. PMID: 12186897 Free PMC article. - DC-SIGN (dendritic cell-specific ICAM-grabbing non-integrin) and DC-SIGN-related (DC-SIGNR): friend or foe?
Soilleux EJ. Soilleux EJ. Clin Sci (Lond). 2003 Apr;104(4):437-46. Clin Sci (Lond). 2003. PMID: 12653690 Review. - DC-SIGN, a dentritic cell-specific HIV-1 receptor present in placenta that infects T cells in trans-a review.
Geijtenbeek TB, van Vliet SJ, van Duijnhoven GC, Figdor CG, van Kooyk Y. Geijtenbeek TB, et al. Placenta. 2001 Apr;22 Suppl A:S19-23. doi: 10.1053/plac.2001.0674. Placenta. 2001. PMID: 11312623 Review.
Cited by
- Transmitted/founder and chronic subtype C HIV-1 use CD4 and CCR5 receptors with equal efficiency and are not inhibited by blocking the integrin α4β7.
Parrish NF, Wilen CB, Banks LB, Iyer SS, Pfaff JM, Salazar-Gonzalez JF, Salazar MG, Decker JM, Parrish EH, Berg A, Hopper J, Hora B, Kumar A, Mahlokozera T, Yuan S, Coleman C, Vermeulen M, Ding H, Ochsenbauer C, Tilton JC, Permar SR, Kappes JC, Betts MR, Busch MP, Gao F, Montefiori D, Haynes BF, Shaw GM, Hahn BH, Doms RW. Parrish NF, et al. PLoS Pathog. 2012;8(5):e1002686. doi: 10.1371/journal.ppat.1002686. Epub 2012 May 31. PLoS Pathog. 2012. PMID: 22693444 Free PMC article. - L-SIGN (CD209L) and DC-SIGN (CD209) mediate transinfection of liver cells by hepatitis C virus.
Cormier EG, Durso RJ, Tsamis F, Boussemart L, Manix C, Olson WC, Gardner JP, Dragic T. Cormier EG, et al. Proc Natl Acad Sci U S A. 2004 Sep 28;101(39):14067-72. doi: 10.1073/pnas.0405695101. Epub 2004 Sep 15. Proc Natl Acad Sci U S A. 2004. PMID: 15371595 Free PMC article. - Unraveling the transcriptional determinants of liver sinusoidal endothelial cell specialization.
de Haan W, Øie C, Benkheil M, Dheedene W, Vinckier S, Coppiello G, Aranguren XL, Beerens M, Jaekers J, Topal B, Verfaillie C, Smedsrød B, Luttun A. de Haan W, et al. Am J Physiol Gastrointest Liver Physiol. 2020 Apr 1;318(4):G803-G815. doi: 10.1152/ajpgi.00215.2019. Epub 2020 Mar 2. Am J Physiol Gastrointest Liver Physiol. 2020. PMID: 32116021 Free PMC article. - The pathogen receptor liver and lymph node sinusoidal endotelial cell C-type lectin is expressed in human Kupffer cells and regulated by PU.1.
Domínguez-Soto A, Aragoneses-Fenoll L, Gómez-Aguado F, Corcuera MT, Clária J, García-Monzón C, Bustos M, Corbí AL. Domínguez-Soto A, et al. Hepatology. 2009 Jan;49(1):287-96. doi: 10.1002/hep.22678. Hepatology. 2009. PMID: 19111020 Free PMC article. - Mapping the distinctive populations of lymphatic endothelial cells in different zones of human lymph nodes.
Park SM, Angel CE, McIntosh JD, Mansell C, Chen CJ, Cebon J, Dunbar PR. Park SM, et al. PLoS One. 2014 Apr 14;9(4):e94781. doi: 10.1371/journal.pone.0094781. eCollection 2014. PLoS One. 2014. PMID: 24733110 Free PMC article.
References
- Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. Science. 1996;272:1955–1958. - PubMed
- Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, et al. Cell. 1996;85:1135–1148. - PubMed
- Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Marzio P D, Marmon S, Sutton R E, Hill C M, et al. Nature (London) 1996;381:661–666. - PubMed
- Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. Cell. 1996;85:1149–1158. - PubMed
- Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, et al. Nature (London) 1996;381:667–673. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous