Glomeruloid microvascular proliferation follows adenoviral vascular permeability factor/vascular endothelial growth factor-164 gene delivery - PubMed (original) (raw)
Glomeruloid microvascular proliferation follows adenoviral vascular permeability factor/vascular endothelial growth factor-164 gene delivery
C Sundberg et al. Am J Pathol. 2001 Mar.
Abstract
Glomeruloid bodies are a defining histological feature of glioblastoma multiforme and some other tumors and vascular malformations. Little is known about their pathogenesis. We injected a nonreplicating adenoviral vector engineered to express vascular permeability factor/vascular endothelial growth factor-164 (VPF/VEGF(164)) into the ears of athymic mice. This vector infected local cells that strongly expressed VPF/VEGF(164) mRNA for 10 to 14 days, after which expression gradually declined. Locally expressed VPF/VEGF(164) induced an early increase in microvascular permeability, leading within 24 hours to edema and deposition of extravascular fibrin; in addition, many pre-existing microvessels enlarged to form thin-walled, pericyte-poor, "mother" vessels. Glomeruloid body precursors were first detected at 3 days as focal accumulations of rapidly proliferating cells in the endothelial lining of mother vessels, immediately adjacent to cells expressing VPF/VEGF(164). Initially, glomeruloid bodies were comprised of endothelial cells but subsequently pericytes and macrophages also participated. As they enlarged by endothelial cell and pericyte proliferation, glomeruloid bodies severely compromised mother vessel lumens and blood flow. Subsequently, as VPF/VEGF(164) expression declined, glomeruloid bodies devolved throughout a period of weeks by apoptosis and reorganization into normal-appearing microvessels. These results provide the first animal model for inducing glomeruloid bodies and indicate that VPF/VEGF(164) is sufficient for their induction and necessary for their maintenance.
Figures
Figure 1.
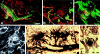
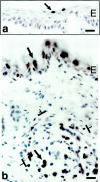
Primitive GBs developing in mother vessels 3 to 4 days after injection of adeno-vpf/vegf into ears of athymic mice. a–c: One-micron-thick, Giemsa-stained Epon sections illustrating focal accumulations of large, primitive cells (arrows) in endothelial cell lining of mother vessels that subsequently develop into GBs. d–g: Immunohistochemical staining demonstrates that primitive precursor cells (black arrows) bear endothelial cell markers (CD-31) (d), relatively increased staining for VEGFR-2 (e), but lack pericyte markers (α-SMA) (f). White arrow in f indicates α-SMA-positive pericytes just peripheral to primitive GBs (black arrow). Staining for basement membrane proteins, decreased or lost during the course of mother vessel formation, is now increased in intensity (entactin) (g). m, mother vessels; lip, osmophilic, lipid-filled cell. Scale bars, 25 μm.
Figure 2.
Course of GB development and devolution in 1-μm Giemsa-stained, Epon sections. a and b: Primitive GBs develop as focal nodules (between brackets) as the result of cell proliferation in the wall of a mother vessel (m) and extend both into the lumen and out into the extravascular connective tissue. Note intimate association with lipid-containing cells (lip). c–e: Maturing GBs encroach on mother vessels, reducing their single large lumens into two or more much smaller lumens (arrows) or obliterating them altogether (e). Note centrally placed, osmophilic, lipid-containing cell (lip) in e. f: Devolving GB has begun to reorganize into normal-appearing microvessels. One newly formed microvessel has acquired a lumen containing red blood cells (white arrow) whereas lumens are just beginning to form in two others (black arrows). g and h: End-stage GB is transformed into relatively normal microvessels (black arrows in g). Residual lipid-containing cells in h (black arrows), much smaller than at earlier stages of GB evolution (compare with a, b, and e), are immediately adjacent to each of three newly formed microvessels. Scale bars, 25 μm.
Figure 3.
a–d: Confocal microscopy of mature GBs. Those in a–c are from mice injected intravenously with both FITC-D (MW, 2,000,000) and TRITC-D (MW, 70,000) 15 minutes before sacrifice. In a and b, GB (white brackets) are perfused with TRITC-D (red), but not with the larger FITC-D (green) that is confined to mother vessels (m) and other larger vessels that feed GBs. In c, mother vessels (two of which are labeled) stain with both dextrans but a GB (asterisk) does not, indicating lack of perfusion with either FITC-D or TRITC-D. d: Immunofluorescent staining of a GB with the basement membrane marker, entactin, illustrates multiple afferent and efferent vessels supplying a GB (white brackets). e and f:. Whole mounts of ear from a mouse perfused with black ink. Note multiple mature GBs (white arrows) supplied by multiple afferent and efferent vessels connected to mother vessels (m). In f, a GB links two mother vessels. Scale bars, 50 μm.
Figure 4.
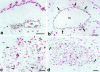
In situ hybridization illustrating cellular VPF/VEGF mRNA expression at various times after injection of adeno-vpf/vegf into the ears of athymic mice. a and b: At 18 hours, scattered cells in dermis (some indicated by black arrows) express large amounts of VPF/VEGF mRNA. b: Some of the positive cells are immediately adjacent to a mother vessel (m). c–e: At 4 days, numerous VPF/VEGF-positive cells are observed deep in the dermis just above the ear cartilage (cart), the location where GBs most commonly form. Note strongly positive cells immediately adjacent to mother vessels (m). e: VPF/VEGF-expressing cells are in immediate proximity to a primitive GB (arrows), developing in a deep dermal mother vessel (m) adjacent to ear cartilage (cart). f: Eight-day reaction illustrating VPF/VEGF-expressing cells in centers of several maturing GBs (one indicated with an arrow). g: At 14 days, several VPF/VEGF-expressing cells persist within GBs (brackets) but average intensity is somewhat reduced in comparison with 8 days. h: Further reduction in numbers and staining intensity of VPF/VEGF-expressing cells at 21 days. A residual positive cell is placed centrally within a GB (brackets). i: By 35 days, VPF/VEGF mRNA was no longer detectably expressed and GBs had begun to devolve into normal microvessels. d and f: Phase contrast microscopy; all others, bright field. Scale bars, 25 μm.
Figure 5.
In situ hybridization for expression of VPF/VEGF receptors in adeno-vpf/vegf injected skin. a and b: VEGFR-1 mRNA expression in vascular endothelium of mother vessels (m) at 4 days. E, epidermis. c and d: VEGFR-2 mRNA expression in GBs (brackets) at 10 days (c) and 21 days (d). Scale bars, 25 μm.
Figure 6.
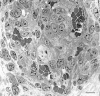
Immunohistochemical characterization of mature GBs (7 to 10 days). a: CD-31-positive cells (putative endothelial cells) continue to predominate. b and c: α-SMA staining of pericytes in vessels of normal skin (arrows in b) and in a mature GB (c) where they are intermingled with endothelial cells. d: F40/80-positive cells (macrophages) are present at the GB periphery (arrows). e and f: Antibodies to entactin (and other basement membrane proteins, not shown) stain basement membranes of vessels and nerves in normal skin (e) and are abundantly deposited but in a disorganized manner in mature GBs (f). L, vascular lumen. Scale bars, 25 μm.
Figure 7.
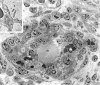
Electron micrograph of a maturing GB. GB appears as a nodular structure with a centrally placed lipid-containing cell (lip) and comprised of several different cell types: E, endothelial cells; P, pericytes; lip, lipid-containing cell with a cytoplasmic lipid body, LB; MO, monocyte; F, fibroblasts. Cells were identified by examination of this same field at higher magnification, using standard electron microscopic criteria. Multiple small vascular lumens (L), many but not all containing red blood cells, are lined by endothelial cells; lesser numbers of pericytes are scattered in between. Scale bar, 10 μm.
Figure 8.
Electron micrograph illustrating a somewhat more mature GB, again centered about a large lipid-containing cell (lip). Several small erythrocyte-containing vascular lumens persist (one marked L). Cell code is the same as that in Figure 7 ▶ with the addition of the following: M, macrophages; Ly, lymphocytes. Individual cells were identified by standard electron microscopic criteria after examining this same field at higher magnification. Inset illustrates multiple layers of basal lamina (asterisk) deposited between luminal endothelial cells and pericytes, a finding also typical of the GBs of glioblastomas. Scale bar, 10 μm; inset bar, 1 μm.
Figure 9.
Early (a, c, and e) and later stages (b, d, and f) of GB devolution into daughter microvessels. a and b: CD-31 staining demonstrates endothelial cells forming discrete clusters that are separated from each other by nonstaining cells (for the most part pericytes). c and d: Pericytes stained with antibodies to NG2 envelop endothelial cells that have begun to form lumens (L). e and f: Entactin staining for basement membrane. g–i: A GB undergoing devolution into daughter microvessels is illustrated by double-fluorescent staining for NG2-expressing pericytes (red, g), basement membrane entactin (green, h), and composite (i). Individual daughter vessels (white arrows) have separated from the main GB cell mass (asterisk). Scale bars, 12.5 μm (a–f) and 25 μm (g–i).
Figure 10.
TUNEL staining to demonstrate apoptotic cells (some indicated by arrows). a: Low-level apoptosis in normal mouse ear epidermis. b: Extensive apoptosis in the formerly hyperplastic epidermal ear skin and in a GB (brackets) at 21 days after ear injection with adeno-vpf/vegf. E, epidermis. Scale bars, 25 μm.
Figure 11.
a and b: Immunohistochemical staining for pericytes in devolving GB. Pericytes (arrows) strongly express α-SMA (a) and NG2 (b) at residual points of interconnection between newly formed daughter vessels. c: Trichrome stain illustrates deposition of fibrillar collagen around newly formed daughter vessels. L, vascular lumens. Scale bars, 12.5 μm.
Figure 12.
Schematic drawing of GB evolution and devolution. Day 0: Normal microvessel lined by endothelial cells, pericytes, and basal lamina. Day 1: One day after injection of adeno-vpf/vegf precursor microvessel has enlarged greatly as the result of pericyte detachment and basal lamina degradation to form a mother vessel (m). A VPF/VEGF-expressing cell (VPC) lies immediately adjacent. Day 2: Proliferation of mother vessel endothelium first noted. Days 3 to 4: Proliferating endothelial cells extend outside the mother vessel and cluster around a VPC. Basal lamina components are deposited in excessive and disorganized multilayers. Pericytes begin to proliferate. Days 7 to 10: GB expansion as the result of cell division has compromised original mother vessel lumen, reducing it here into two smaller channels. Pericytes are apparently intermingled among endothelial cells. Macrophages accumulate at GB periphery. Day 14: Earliest stages of GB devolution as VPF/VEGF expression by VPC is on the wane. Pericytes are more numerous and surround clusters of endothelial cells that have now formed obvious vascular lumens. Day 21: Later stage of GB devolution with greatly reduced VPF/VEGF expression and better definition of individually forming microvessels. Day 28: Newly formed microvessels have begun to separate from each other but some remain linked by pericytes that strongly express contractile proteins such as α-SMA. Small amounts of fibrillar collagen are deposited. Days 35 to 90: GBs are completely replaced by normal-appearing microvessels interspersed between moderate amounts of newly deposited fibrillar collagen.
Comment in
- Glomeruloid microvascular proliferation orchestrated by VPF/VEGF: a new world of angiogenesis research.
Brat DJ, Van Meir EG. Brat DJ, et al. Am J Pathol. 2001 Mar;158(3):789-96. doi: 10.1016/S0002-9440(10)64025-4. Am J Pathol. 2001. PMID: 11238026 Free PMC article. Review. No abstract available. - Plexiform lesion in severe pulmonary hypertension: association with glomeruloid lesion.
Tuder RM, Voelkel NF. Tuder RM, et al. Am J Pathol. 2001 Jul;159(1):382-3. doi: 10.1016/S0002-9440(10)61705-1. Am J Pathol. 2001. PMID: 11438486 Free PMC article. No abstract available.
Similar articles
- Strong expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in ovarian borderline and malignant neoplasms.
Abu-Jawdeh GM, Faix JD, Niloff J, Tognazzi K, Manseau E, Dvorak HF, Brown LF. Abu-Jawdeh GM, et al. Lab Invest. 1996 Jun;74(6):1105-15. Lab Invest. 1996. PMID: 8667614 - Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in adenocarcinomas of the gastrointestinal tract.
Brown LF, Berse B, Jackman RW, Tognazzi K, Manseau EJ, Senger DR, Dvorak HF. Brown LF, et al. Cancer Res. 1993 Oct 1;53(19):4727-35. Cancer Res. 1993. PMID: 8402650 - Treatment for malignant pleural effusion of human lung adenocarcinoma by inhibition of vascular endothelial growth factor receptor tyrosine kinase phosphorylation.
Yano S, Herbst RS, Shinohara H, Knighton B, Bucana CD, Killion JJ, Wood J, Fidler IJ. Yano S, et al. Clin Cancer Res. 2000 Mar;6(3):957-65. Clin Cancer Res. 2000. PMID: 10741721 - Vascular permeability factor/vascular endothelial growth factor: a multifunctional angiogenic cytokine.
Brown LF, Detmar M, Claffey K, Nagy JA, Feng D, Dvorak AM, Dvorak HF. Brown LF, et al. EXS. 1997;79:233-69. doi: 10.1007/978-3-0348-9006-9_10. EXS. 1997. PMID: 9002222 Review. - Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis.
Dvorak HF, Brown LF, Detmar M, Dvorak AM. Dvorak HF, et al. Am J Pathol. 1995 May;146(5):1029-39. Am J Pathol. 1995. PMID: 7538264 Free PMC article. Review.
Cited by
- Expression of vascular endothelial growth factor is coordinately regulated by the activin-like kinase receptors 1 and 5 in endothelial cells.
Shao ES, Lin L, Yao Y, Boström KI. Shao ES, et al. Blood. 2009 Sep 3;114(10):2197-206. doi: 10.1182/blood-2009-01-199166. Epub 2009 Jun 8. Blood. 2009. PMID: 19506300 Free PMC article. - Dynamic MR imaging for functional vascularization depends on tissue factor signaling in glioblastoma.
Chen X, Xie T, Fang J, Xue W, Kang H, Tong H, Guo Y, Zhang B, Wang S, Yang Y, Zhang W. Chen X, et al. Cancer Biol Ther. 2018 May 4;19(5):416-426. doi: 10.1080/15384047.2018.1423924. Epub 2018 Feb 6. Cancer Biol Ther. 2018. PMID: 29333924 Free PMC article. - Pathological angiogenesis is induced by sustained Akt signaling and inhibited by rapamycin.
Phung TL, Ziv K, Dabydeen D, Eyiah-Mensah G, Riveros M, Perruzzi C, Sun J, Monahan-Earley RA, Shiojima I, Nagy JA, Lin MI, Walsh K, Dvorak AM, Briscoe DM, Neeman M, Sessa WC, Dvorak HF, Benjamin LE. Phung TL, et al. Cancer Cell. 2006 Aug;10(2):159-70. doi: 10.1016/j.ccr.2006.07.003. Cancer Cell. 2006. PMID: 16904613 Free PMC article. - The L6 protein TM4SF1 is critical for endothelial cell function and tumor angiogenesis.
Shih SC, Zukauskas A, Li D, Liu G, Ang LH, Nagy JA, Brown LF, Dvorak HF. Shih SC, et al. Cancer Res. 2009 Apr 15;69(8):3272-7. doi: 10.1158/0008-5472.CAN-08-4886. Epub 2009 Apr 7. Cancer Res. 2009. PMID: 19351819 Free PMC article. - In pursuit of new anti-angiogenic therapies for cancer treatment.
Cai J, Han S, Qing R, Liao D, Law B, Boulton ME. Cai J, et al. Front Biosci (Landmark Ed). 2011 Jan 1;16(3):803-14. doi: 10.2741/3721. Front Biosci (Landmark Ed). 2011. PMID: 21196204 Free PMC article. Review.
References
- Pettersson A, Nagy JA, Brown LF, Sundberg C, Morgan E, Jungles S, Carter R, Krieger JE, Manseau EJ, Harvey VS, Eckelhoefer IA, Feng D, Dvorak AM, Mulligan RC, Dvorak HF: Heterogeneity of the angiogenic response induced in different normal adult tissues by vascular permeability factor/vascular endothelial growth factor. Lab Invest 2000, 80:99-115 - PubMed
- Rojiani AM, Dorovini-Zis K: Glomeruloid vascular structures in glioblastoma multiforme: an immunohistochemical and ultrastructural study. J Neurosurg 1996, 85:1078-1084 - PubMed
- McLendon R, Enterline D, Tien R, Thorstad W, Bruner J: Tumors of central neuroepithelial origin. Bigner D McLendon R Bruner J eds. Russell and Rubinstein’s Pathology of Tumors of the Nervous System, ch 9. 1998, :pp 307-571 Oxford University Press, New York
- Blaker H, Dragoje S, Laissue JA, Otto HF: Pericardial involvement by thymomas. Entirely intrapericardial thymoma and a pericardial metastasis of thymoma with glomeruloid vascular proliferations. Pathol Oncol Res 1999, 5:160-163 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA050453/CA/NCI NIH HHS/United States
- AI-33372/AI/NIAID NIH HHS/United States
- R01 AI033372/AI/NIAID NIH HHS/United States
- HL-59316/HL/NHLBI NIH HHS/United States
- CA-50453/CA/NCI NIH HHS/United States
- AI-44066/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials