A multistep damage recognition mechanism for global genomic nucleotide excision repair - PubMed (original) (raw)
A multistep damage recognition mechanism for global genomic nucleotide excision repair
K Sugasawa et al. Genes Dev. 2001.
Abstract
A mammalian nucleotide excision repair (NER) factor, the XPC-HR23B complex, can specifically bind to certain DNA lesions and initiate the cell-free repair reaction. Here we describe a detailed analysis of its binding specificity using various DNA substrates, each containing a single defined lesion. A highly sensitive gel mobility shift assay revealed that XPC-HR23B specifically binds a small bubble structure with or without damaged bases, whereas dual incision takes place only when damage is present in the bubble. This is evidence that damage recognition for NER is accomplished through at least two steps; XPC-HR23B first binds to a site that has a DNA helix distortion, and then the presence of injured bases is verified prior to dual incision. Cyclobutane pyrimidine dimers (CPDs) were hardly recognized by XPC-HR23B, suggesting that additional factors may be required for CPD recognition. Although the presence of mismatched bases opposite a CPD potentiated XPC-HR23B binding, probably due to enhancement of the helix distortion, cell-free excision of such compound lesions was much more efficient than expected from the observed affinity for XPC-HR23B. This also suggests that additional factors and steps are required for the recognition of some types of lesions. A multistep mechanism of this sort may provide a molecular basis for ensuring the high level of damage discrimination that is required for global genomic NER.
Figures
Figure 1
Defined DNA substrates used in this study. Arrows indicate major incision sites in each AAF-damaged substrate, which were determined by the primer extension method.
Figure 2
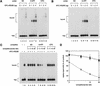
Gel mobility shift analysis of XPC–HR23B binding to a single UV photolesion. (A,B) Indicated amounts of purified XPC–HR23B were incubated with 3.5 fmole each of the 32P-labeled DNA fragment containing a 6-4PP or a CPD, or the cognate nondamaged (ND) control fragment. The binding reactions were performed in the absence (A) or presence (B) of a small amount (0.5 ng) of covalently closed circular plasmid DNA. The resulting DNA–protein complexes were fixed with glutaraldehyde and separated by PAGE. (C) A competition experiment using the labeled 6-4PP probe (3.5 fmole), which was incubated with (+) or without (−) 2 ng of XPC–HR23B and various amounts of cold competitor DNA fragments as indicated. (D) The amount of the labeled probe complexed with XPC–HR23B was calculated for each lane in C, and expressed as a percentage of the control (lane 2) without competitors. The mean values and standard errors were calculated from two or three independent experiments.(●) ND; (▴) 6-4PP; (▪) CPD.
Figure 3
Damage-independent binding of XPC–HR23B to a small bubble. (A) Gel mobility shift assay using the 32P-labeled probe with or without the AAF adduct and a bubble. (B) A competition experiment involving the 32P-labeled 6-4PP probe (3.5 fmole) and 2 ng of XPC–HR23B. Various amounts of nonlabeled competitor DNA fragments were included in the binding reactions as indicated. (C) Quantitative representation of the competition profiles. Complex formation in each lane of B was quantified as in Fig. 2D. The mean values and standard errors were calculated from at least two independent experiments. The competition profile by 6-4PP obtained in Fig. 2D is superimposed. (○) B0(AAF−); (●) B0(AAF+); (□) B3(AFF−); (▪) B3(AFF+); (▴) 6-4PP. (D) A competition experiment using the 32P-labeled B5(AAF +) probe and 1 ng of XPC–HR23B.
Figure 4
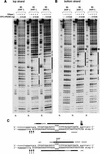
DNase I footprinting analysis of XPC–HR23B binding to a 3-base bubble. (A,B) The indicated substrates were 5′-end-labeled for top strands (A) or bottom strands (B), and subjected to the DNase I footprinting assay using various amounts of XPC–HR23B as indicated. The digested DNA samples were subjected to denaturing PAGE followed by autoradiography. The Maxam-Gilbert G ladder prepared from each probe was loaded in parallel, where indicated, below the gels. The position of the bubble in each substrate is shown by an asterisk. (C) A schematic representation of the protection patterns for the B3 substrates. In each panel, strongly and weakly protected regions are shown by solid and shaded bars, respectively. The sites that became hypersensitive to DNase I upon binding are indicated by arrowheads (A,B) or arrows (C). Size of the arrows in C corresponds to the observed degree of hypersensitivity.
Figure 5
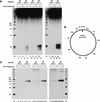
Binding of XPC–HR23B is not sufficient for the NER incision, but damage is also required. (A) The indicated substrates were internally labeled with 32P and used for the dual incision assay involving the XP-C whole cell extract and various amounts of XPC–HR23B. The DNA samples were subjected to denaturing PAGE followed by autoradiography. (M) 32P-labeled 25-bp ladder. (B) A map of _Hae_III-cutting sites in the DNA substrates, where the size of each fragment (in base pairs) is indicated. (C) The nonlabeled closed circular DNA substrates indicated were incubated in the cell-free NER reactions including the XP-C whole cell extract, various amounts of XPC–HR23B, and aphidicolin. The purified DNA samples were subjected to gap-filling DNA synthesis with T4 DNA polymerase and radiolabeled dNTPs, digestion with _Hae_III, and nondenaturing PAGE followed by autoradiography.
Figure 6
Presence of mismatched bases opposite CPD enhances the binding affinity for XPC–HR23B as well as the damage excision efficiency. (A) Gel mobility shift assay using the indicated probes and various amounts of XPC–HR23B. (B) A competition experiment involving the 32P-labeled 6-4PP probe (3.5 fmole) and 2 ng of XPC–HR23B. Various amounts of nonlabeled competitor DNA fragments were included in the binding reactions as indicated. (C) Quantitative representation of the competition profiles. The mean values and standard errors were calculated from at least two independent experiments. The competition profile of 6-4PP is superimposed. (▪) CPD; (○) CPD(GA); (●) CPD(GG); (▴) 6-4PP. (D) The indicated, internally labeled substrates were assayed for the NER incision in the XP-C whole cell extract supplemented with various amounts of XPC–HR23B. A part of the autoradiograph showing the dual incision products is presented. (M) 32P-labeled 25-bp ladder.
Figure 7
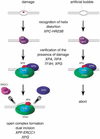
A model for two-step damage recognition in global genomic NER. XPC–HR23B first recognizes the site where helix distortion is induced. Other NER factors, involving TFIIH, XPA, XPG, and RPA, may be then recruited to the suspected site, where they somehow verify the presence of a lesion that is suitable to be handled by NER. If there is a lesion (left), the pre-incision complex containing fully opened DNA would be assembled, leading to dual incision by XPF–ERCC1 and XPG. If there is not a lesion (right), the process may be cancelled at a certain stage prior to the open complex assembly.
Similar articles
- Diversity of the damage recognition step in the global genomic nucleotide excision repair in vitro.
Kusumoto R, Masutani C, Sugasawa K, Iwai S, Araki M, Uchida A, Mizukoshi T, Hanaoka F. Kusumoto R, et al. Mutat Res. 2001 Apr 4;485(3):219-27. doi: 10.1016/s0921-8777(00)00082-3. Mutat Res. 2001. PMID: 11267833 - NMR structure of the DNA decamer duplex containing double T*G mismatches of cis-syn cyclobutane pyrimidine dimer: implications for DNA damage recognition by the XPC-hHR23B complex.
Lee JH, Park CJ, Shin JS, Ikegami T, Akutsu H, Choi BS. Lee JH, et al. Nucleic Acids Res. 2004 Apr 30;32(8):2474-81. doi: 10.1093/nar/gkh568. Print 2004. Nucleic Acids Res. 2004. PMID: 15121904 Free PMC article. - A molecular mechanism for DNA damage recognition by the xeroderma pigmentosum group C protein complex.
Sugasawa K, Shimizu Y, Iwai S, Hanaoka F. Sugasawa K, et al. DNA Repair (Amst). 2002 Jan 22;1(1):95-107. doi: 10.1016/s1568-7864(01)00008-8. DNA Repair (Amst). 2002. PMID: 12509299 - Molecular basis for damage recognition and verification by XPC-RAD23B and TFIIH in nucleotide excision repair.
Mu H, Geacintov NE, Broyde S, Yeo JE, Schärer OD. Mu H, et al. DNA Repair (Amst). 2018 Nov;71:33-42. doi: 10.1016/j.dnarep.2018.08.005. Epub 2018 Aug 23. DNA Repair (Amst). 2018. PMID: 30174301 Free PMC article. Review. - Open, repair and close again: chromatin dynamics and the response to UV-induced DNA damage.
Palomera-Sanchez Z, Zurita M. Palomera-Sanchez Z, et al. DNA Repair (Amst). 2011 Feb 7;10(2):119-25. doi: 10.1016/j.dnarep.2010.10.010. Epub 2010 Dec 3. DNA Repair (Amst). 2011. PMID: 21130713 Review.
Cited by
- Molecular architecture and functional dynamics of the pre-incision complex in nucleotide excision repair.
Yu J, Yan C, Paul T, Brewer L, Tsutakawa SE, Tsai CL, Hamdan SM, Tainer JA, Ivanov I. Yu J, et al. Nat Commun. 2024 Oct 1;15(1):8511. doi: 10.1038/s41467-024-52860-y. Nat Commun. 2024. PMID: 39353945 Free PMC article. - Differential processing of RNA polymerase II at DNA damage correlates with transcription-coupled repair syndrome severity.
Gonzalo-Hansen C, Steurer B, Janssens RC, Zhou D, van Sluis M, Lans H, Marteijn JA. Gonzalo-Hansen C, et al. Nucleic Acids Res. 2024 Sep 9;52(16):9596-9612. doi: 10.1093/nar/gkae618. Nucleic Acids Res. 2024. PMID: 39021334 Free PMC article. - Analysis of cytosine deamination events in excision repair sequencing reads reveals mechanisms of incision site selection in NER.
Morledge-Hampton B, Kalyanaraman A, Wyrick JJ. Morledge-Hampton B, et al. Nucleic Acids Res. 2024 Feb 28;52(4):1720-1735. doi: 10.1093/nar/gkad1195. Nucleic Acids Res. 2024. PMID: 38109317 Free PMC article. - Detection of oxaliplatin- and cisplatin-DNA lesions requires different global genome repair mechanisms that affect their clinical efficacy.
Slyskova J, Muniesa-Vargas A, da Silva IT, Drummond R, Park J, Häckes D, Poetsch I, Ribeiro-Silva C, Moretton A, Heffeter P, Schärer OD, Vermeulen W, Lans H, Loizou JI. Slyskova J, et al. NAR Cancer. 2023 Dec 5;5(4):zcad057. doi: 10.1093/narcan/zcad057. eCollection 2023 Dec. NAR Cancer. 2023. PMID: 38058548 Free PMC article. - Differing structures and dynamics of two photolesions portray verification differences by the human XPD helicase.
Fu I, Geacintov NE, Broyde S. Fu I, et al. Nucleic Acids Res. 2023 Dec 11;51(22):12261-12274. doi: 10.1093/nar/gkad974. Nucleic Acids Res. 2023. PMID: 37933861 Free PMC article.
References
- Asahina H, Kuraoka I, Shirakawa M, Morita EH, Miura N, Miyamoto I, Ohtsuka E, Okada Y, Tanaka K. The XPA protein is a zinc metalloprotein with an ability to recognize various kinds of DNA damage. Mutat Res. 1994;315:229–237. - PubMed
- Batty D, Rapic'-Otrin V, Levine AS, Wood RD. Stable binding of human XPC complex to irradiated DNA confers strong discrimination for damaged sites. J Mol Biol. 2000;300:275–290. - PubMed
- Bohr VA, Smith CA, Okumoto DS, Hanawalt PC. DNA repair in active gene: Removal of pyrimidine dimers from the DHFR gene of CHO cells is much more efficient than in the genome overall. Cell. 1985;40:359–369. - PubMed
- Bootsma D, Kraemer KH, Cleaver J, Hoeijmakers JHJ. Nucleotide excision repair syndromes: Xeroderma pigmentosum, Cockayne syndrome and trichothiodystrophy. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic basis of inherited disease. New York, NY: McGraw-Hill Book Co.; 1997.
- Burns J, Guzder S, Sung P, Prakash S, Prakash L. An affinity of human replication protein A for ultraviolet-damaged DNA. J Biol Chem. 1996;271:11607–11610. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources