Accumulation of caveolin in the endoplasmic reticulum redirects the protein to lipid storage droplets - PubMed (original) (raw)
Accumulation of caveolin in the endoplasmic reticulum redirects the protein to lipid storage droplets
A G Ostermeyer et al. J Cell Biol. 2001.
Abstract
Caveolin-1 is normally localized in plasma membrane caveolae and the Golgi apparatus in mammalian cells. We found three treatments that redirected the protein to lipid storage droplets, identified by staining with the lipophilic dye Nile red and the marker protein ADRP. Caveolin-1 was targeted to the droplets when linked to the ER-retrieval sequence, KKSL, generating Cav-KKSL. Cav-DeltaN2, an internal deletion mutant, also accumulated in the droplets, as well as in a Golgi-like structure. Third, incubation of cells with brefeldin A caused caveolin-1 to accumulate in the droplets. This localization persisted after drug washout, showing that caveolin-1 was transported out of the droplets slowly or not at all. Some overexpressed caveolin-2 was also present in lipid droplets. Experimental reduction of cellular cholesteryl ester by 80% did not prevent targeting of Cav-KKSL to the droplets. Cav-KKSL expression did not grossly alter cellular triacylglyceride or cholesteryl levels, although droplet morphology was affected in some cells. These data suggest that accumulation of caveolin-1 to unusually high levels in the ER causes targeting to lipid droplets, and that mechanisms must exist to ensure the rapid exit of newly synthesized caveolin-1 from the ER to avoid this fate.
Figures
Figure 1
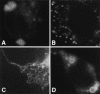
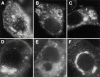
Localization of Cav–KKSL. Cav–KKSL in transiently transfected FRT (A and B) or COS (C and D) cells was visualized by IF.
Figure 3
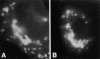
Localization of Cav–ΔN2 and caveolin-2. IF localization of Cav–ΔN2 (A) and caveolin-2 (B) in transiently transfected FRT cells. The Golgi apparatus was overexposed to show the droplets.
Figure 2
Colocalization of Cav–KKSL, ADRP, and Nile red staining. Endogenous ADRP (A) and transiently expressed Cav–KKSL (B) in a COS cell were visualized by IF. C, Merged image of A and B. Nile red stain (D) and IF visualization of Cav–KKSL (E) in a transiently transfected FRT cell. F, Merged image of D and E.
Figure 5
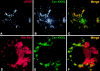
Partial recruitment of caveolin-1 to lipid droplets by Cav–KKSL. FRT cells were transiently cotransfected with Cav–KKSL and HA/Myc-tagged caveolin-1 and double-labeled with anti-caveolin-1 antibodies and Texas-red secondary antibodies (A; to detect both proteins) and anti-HA antibodies and DTAF secondary antibodies (B; to detect the wild-type protein specifically). Arrow, plasma membrane staining; arrowheads, lipid droplets.
Figure 4
Effect of BFA on caveolin-1 localization. FRT cells were transiently transfected with HA/myc-tagged caveolin-1. Cells were either left untreated (A and D) or BFA-treated for 5 h (B, C, E, and F). BFA-treated cells were either fixed immediately (B and E) or were incubated at 37°C for 3 h in normal growth media after washing out BFA before fixation (C and F). Caveolin-1 was visualized by IF (A–C). Golgi apparatus morphology in cells on separate coverslips was monitored using FITC-lens lectin (D–F). Experiments in which caveolin-1 and lens lectin staining were visualized in the same cells gave similar results (not shown).
Figure 6
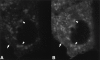
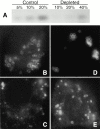
CE depletion does not affect Cav–KKSL localization. A, FRT cells in 100-mm dishes were grown as usual (Control) or depleted of CE as described in Materials and Methods (Depleted). The indicated fraction of the total lipids isolated from each plate was then analyzed by HP-TLC as in Materials and Methods. The charred CE bands are shown. The levels of other lipids were not affected (not shown). B–E, Cav–KKSL in CE-depleted FRT cells transiently expressing Cav–KKSL was visualized by IF. The range of staining patterns was identical to that in control cells processed in parallel (not shown).
Figure 7
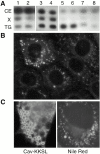
Effect of Cav–KKSL on lipid droplets. A, Lipids from various cell types were separated by HP-TLC and visualized by charring. Lanes 1 and 2 are from a separate plate. Positions of CE, TG, and unidentified lipid X are indicated. Cells used and (percent of cells expressing Cav–KKSL); 1, G418-resistant FRT cells; 2, FRT clone B4 stably expressing Cav–KKSL (19%); 3, transient FRT/vector; 4, transient FRT/Cav–KKSL (32%); 5, COS/vector; 6, COS/Cav–KKSL (36%); 7, HEK 293/vector; 8, HEK 293/Cav–KKSL (53%). Only 10% of the COS cell extracts (lanes 5 and 6) were loaded on the plate. B, Untransfected FRT cells were stained with Nile red. C, COS cells were transiently transfected with Cav–KKSL, detecting the protein by IF (left), or were stained with Nile red (right; a cell with large droplets).
Figure 8
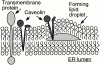
How caveolin-1 might enter lipid droplets. Neutral lipids may accumulate in the interior of the ER bilayer, making a bulge that eventually buds into the cytoplasm to form a droplet surrounded by an ER-derived phospholipid monolayer. This process would drive opposite ER membrane monolayers apart, effectively thickening the hydrophobic portion of the membrane. Most membrane proteins, which contain hydrophilic domains on both sides of the bilayer, could not be accommodated in this environment and would be excluded from the forming droplets. By contrast, caveolin-1 has no luminal hydrophilic domain and could easily diffuse laterally between the ER membrane proper and the monolayer surrounding the nascent droplets.
Comment in
- Caveolin, cholesterol, and lipid droplets?
van Meer G. van Meer G. J Cell Biol. 2001 Mar 5;152(5):F29-34. doi: 10.1083/jcb.152.5.f29. J Cell Biol. 2001. PMID: 11238468 Free PMC article. No abstract available.
Similar articles
- A caveolin dominant negative mutant associates with lipid bodies and induces intracellular cholesterol imbalance.
Pol A, Luetterforst R, Lindsay M, Heino S, Ikonen E, Parton RG. Pol A, et al. J Cell Biol. 2001 Mar 5;152(5):1057-70. doi: 10.1083/jcb.152.5.1057. J Cell Biol. 2001. PMID: 11238460 Free PMC article. - Caveolin-2 is targeted to lipid droplets, a new "membrane domain" in the cell.
Fujimoto T, Kogo H, Ishiguro K, Tauchi K, Nomura R. Fujimoto T, et al. J Cell Biol. 2001 Mar 5;152(5):1079-85. doi: 10.1083/jcb.152.5.1079. J Cell Biol. 2001. PMID: 11238462 Free PMC article. - Role of the hydrophobic domain in targeting caveolin-1 to lipid droplets.
Ostermeyer AG, Ramcharan LT, Zeng Y, Lublin DM, Brown DA. Ostermeyer AG, et al. J Cell Biol. 2004 Jan 5;164(1):69-78. doi: 10.1083/jcb.200303037. J Cell Biol. 2004. PMID: 14709541 Free PMC article. - Getting rid of caveolins: phenotypes of caveolin-deficient animals.
Le Lay S, Kurzchalia TV. Le Lay S, et al. Biochim Biophys Acta. 2005 Dec 30;1746(3):322-33. doi: 10.1016/j.bbamcr.2005.06.001. Epub 2005 Jun 23. Biochim Biophys Acta. 2005. PMID: 16019085 Review. - The caveolin proteins.
Williams TM, Lisanti MP. Williams TM, et al. Genome Biol. 2004;5(3):214. doi: 10.1186/gb-2004-5-3-214. Epub 2004 Mar 1. Genome Biol. 2004. PMID: 15003112 Free PMC article. Review.
Cited by
- Monotopic topology is required for lipid droplet targeting of ancient ubiquitous protein 1.
Stevanovic A, Thiele C. Stevanovic A, et al. J Lipid Res. 2013 Feb;54(2):503-13. doi: 10.1194/jlr.M033852. Epub 2012 Nov 29. J Lipid Res. 2013. PMID: 23197321 Free PMC article. - Secretion and fluid transport mechanisms in the mammary gland: comparisons with the exocrine pancreas and the salivary gland.
McManaman JL, Reyland ME, Thrower EC. McManaman JL, et al. J Mammary Gland Biol Neoplasia. 2006 Oct;11(3-4):249-68. doi: 10.1007/s10911-006-9031-3. J Mammary Gland Biol Neoplasia. 2006. PMID: 17136613 Review. - Caveolae are highly immobile plasma membrane microdomains, which are not involved in constitutive endocytic trafficking.
Thomsen P, Roepstorff K, Stahlhut M, van Deurs B. Thomsen P, et al. Mol Biol Cell. 2002 Jan;13(1):238-50. doi: 10.1091/mbc.01-06-0317. Mol Biol Cell. 2002. PMID: 11809836 Free PMC article. - Caveolin, cholesterol, and lipid droplets?
van Meer G. van Meer G. J Cell Biol. 2001 Mar 5;152(5):F29-34. doi: 10.1083/jcb.152.5.f29. J Cell Biol. 2001. PMID: 11238468 Free PMC article. No abstract available. - Nile Red Quantifier: a novel and quantitative tool to study lipid accumulation in patient-derived circulating monocytes using confocal microscopy.
Schnitzler JG, Bernelot Moens SJ, Tiessens F, Bakker GJ, Dallinga-Thie GM, Groen AK, Nieuwdorp M, Stroes ESG, Kroon J. Schnitzler JG, et al. J Lipid Res. 2017 Nov;58(11):2210-2219. doi: 10.1194/jlr.D073197. Epub 2017 Sep 28. J Lipid Res. 2017. PMID: 28972117 Free PMC article.
References
- Arni S., Keitbaugh S.A., Ostermeyer A.G., Brown D.A. Association of GAP-43 with detergent-resitant membranes requires two palmitoylated cysteine residues. J. Biol. Chem. 1998;273:28478–28485. - PubMed
- Bednarek S.Y., Ravazzola M., Hosobuchi M., Amherdt M., Perrelet A., Schekman R., Orci L. COPI- and COPII-coated vesicles bud directly from the endoplasmic reticulum in yeast. Cell. 1995;83:1183–1196. - PubMed
- Brasaemle D.L., Rubin B., Harten I.A., Gruia-Gray J., Kimmel A.R., Londos C. Perilipin A increases triacylglycerol storage by decreasing the rate of triacylglycerol hydrolysis. J. Biol. Chem. 2000;275:38486–38493. - PubMed
- Brown D.A., Rose J.K. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials