ICP0 is required for efficient reactivation of herpes simplex virus type 1 from neuronal latency - PubMed (original) (raw)
ICP0 is required for efficient reactivation of herpes simplex virus type 1 from neuronal latency
W P Halford et al. J Virol. 2001 Apr.
Abstract
Relative to wild-type herpes simplex virus type 1 (HSV-1), ICP0-null mutant viruses reactivate inefficiently from explanted, latently infected mouse trigeminal ganglia (TG), indicating that ICP0 is not essential for reactivation but plays a central role in enhancing the efficiency of reactivation. The validity of these findings has been questioned, however, because the replication of ICP0-null mutants is impaired in animal models during the establishment of latency, such that fewer mutant genomes than wild-type genomes are present in latently infected mouse TG. Therefore, the reduced number of mutant viral genomes available to reactivate, rather than mutations in the ICP0 gene per se, may be responsible for the reduced reactivation efficiency of ICP0-null mutants. We have recently demonstrated that optimization of the size of the ICP0 mutant virus inoculum and transient immunosuppression of mutant-infected mice with cyclophosphamide can be used to establish wild-type levels of ICP0-null mutant genomes in latently infected TG (W. P. Halford and P. A. Schaffer, J. Virol. 74:5957-5967, 2000). Using this procedure to equalize mutant and wild-type genome numbers, the goal of the present study was to determine if, relative to wild-type virus, the absence of ICP0 function in two ICP0-null mutants, n212 and 7134, affects reactivation efficiency from (i) explants of latently infected TG and (ii) primary cultures of latently infected TG cells. Although equivalent numbers of viral genomes were present in TG of mice latently infected with either wild-type or mutant viruses, reactivation of n212 and 7134 from heat-stressed TG explants was inefficient (31 and 37% reactivation, respectively) relative to reactivation of wild-type virus (KOS) (95%). Similarly, n212 and 7134 reactivated inefficiently from primary cultures of dissociated TG cells plated directly after removal from the mouse (7 and 4% reactivation, respectively), relative to KOS (60% reactivation). The efficiency and kinetics of reactivation of KOS, n212, and 7134 from cultured TG cells (treated with acyclovir to facilitate the establishment of latency) in response to heat stress or superinfection with a nonreplicating HSV-1 ICP4(-) mutant, n12, were compared. Whereas heat stress induced reactivation of KOS from 69% of latently infected TG cell cultures, reactivation of n212 and 7134 was detected in only 1 and 7% of cultures, respectively. In contrast, superinfection with the ICP4(-) virus, which expresses high levels of ICP0, resulted in the production of infectious virus in nearly 100% of cultures latently infected with KOS, n212, or 7134 within 72 h. Thus, although latent mutant viral genome loads were equivalent to that of wild-type virus, in the absence of ICP0, n212 and 7134 reactivated inefficiently from latently infected TG cells during culture establishment and following heat stress. Collectively, these findings demonstrate that ICP0 is required to induce efficient reactivation of HSV-1 from neuronal latency.
Figures
FIG. 1
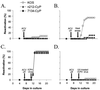
Viral genome loads and reactivation efficiencies of KOS, _n_212, and 7134 from latently infected TG explants. (A) Viral genome loads in TG latently infected with KOS, _n_212, or 7134 and derived from untreated or CyP-treated mice (n = 5 TG per group), as determined by competitive PCR assay. The dashed line indicates the lower limit of the quantitative range of the competitive PCR. Measurements below this line are not significantly different from background. Error bars indicate SEMs. (B) Efficiencies of reactivation from explanted TG latently infected with KOS, _n_212, or 7134 (n = 12 TG per group) and heat stressed at the time of explantation. On days 2 to 11 postexplant, reactivation was assessed by the presence or absence of infectious virus in cell culture medium. On day 12, reactivation was detected by the presence of infectious virus in homogenates of TG explants.
FIG. 2
Numbers of KOS, _n_212, and 7134 infectious centers detected in latently infected TG. Infectious centers were initially determined in pairs of TG derived from untreated and CyP-treated mice that were either uninfected (UI) (n = 3 TG pairs) or latently infected with KOS, _n_212, or 7134 (n = 8 TG pairs per group), and the total numbers were divided by 2. The horizontal bars in each column indicate the average number of infectious centers observed in individual TG of each treatment group.
FIG. 3
Viral genome loads and reactivation efficiencies of KOS, _n_212, and 7134 from primary cultures of latently infected TG cells. (A) Viral genome loads in aliquots of TG cells latently infected with KOS, _n_212, or 7134 derived from untreated or CyP-treated mice (n = 4 1-ml aliquots per group), as determined by competitive PCR. The dashed line indicates the lower limit of the quantitative range of the competitive PCR. Measurements below this line are not significantly different from background. Error bars indicate SEMs. (B) Efficiencies of reactivation from TG cells latently infected with KOS, _n_212, or 7134 (n = 24 TG cell cultures per group). On days 2 to 11 after culture establishment, reactivation was assessed by the presence or absence of infectious virus in culture medium. On day 12, reactivation was detected by the presence of infectious virus in TG cell culture suspensions prepared by sequential freeze-thawing and sonication.
FIG. 4
Reactivation efficiencies of KOS, _n_212, and 7134 from latently infected TG cell cultures that were heat stressed or superinfected with an ICP4− mutant virus. At the time of cell culture preparation, cultures were incubated in medium containing 200 μM ACV to inhibit virus replication. On day 7, ACV-containing medium was replaced with ACV-free medium. On day 11, cultures were left untreated (A), subjected to heat stress (B), superinfected with an ICP4− virus (C), or superinfected with a UV-inactivated ICP4− virus (D). On day 4 and days 7 to 21 after culture establishment, reactivation was assessed by the presence or absence of infectious virus in the culture medium.
FIG. 5
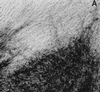
Foci of HSV antigen expression in TG cell cultures infected with KOS (A), _n_212 (B), or 7134 (C) on day 10 after heat stress. Magnification, ×10. Viral antigens were visualized using horseradish peroxidase-conjugated polyclonal rabbit antibody to HSV-1 and the substrate aminoethylcarbazole.
FIG. 5
Foci of HSV antigen expression in TG cell cultures infected with KOS (A), _n_212 (B), or 7134 (C) on day 10 after heat stress. Magnification, ×10. Viral antigens were visualized using horseradish peroxidase-conjugated polyclonal rabbit antibody to HSV-1 and the substrate aminoethylcarbazole.
FIG. 5
Foci of HSV antigen expression in TG cell cultures infected with KOS (A), _n_212 (B), or 7134 (C) on day 10 after heat stress. Magnification, ×10. Viral antigens were visualized using horseradish peroxidase-conjugated polyclonal rabbit antibody to HSV-1 and the substrate aminoethylcarbazole.
FIG. 6
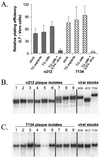
Stability of phenotypes and genotypes of _n_212 and 7134 reactivation isolates relative to viral stocks used for ocular inoculation. (A) Ratios of relative plating efficiencies (L7 versus Vero cells) of the _n_212 and 7134 viral stocks used to inoculate mice (n = 3 independent tests per virus), of isolates obtained from reactivating TG latently infected with _n_212 and 7134 (n = 6 isolates per group), of isolates obtained from TG cell cultures that reactivated during culture establishment or following heat stress (n = 6 isolates per group), and of isolates obtained from latently infected TG cell cultures superinfected with ICP4− virus (n = 17 isolates per group). Error bars indicate SEMs. (B and C) Southern blot analysis of viral DNAs from individual reactivation isolates of _n_212 (B) and 7134 (C) compared with _n_212 and 7134 virus stocks used for ocular inoculation. Total DNA (3 μg) from _n_212-infected L7 cells was digested with _Nco_I and _Spe_I, and total DNA from 7134-infected L7 cells was digested with _Not_I. Following eletrophoretic separation of DNA fragments on a 0.8% agarose gel and transfer of separated fragments to nitrocellulose, blots were hybridized to an ICP0-specific oligonucleotide. From left to right, the samples on each blot include DNAs of three independent reactivation isolates obtained from latently infected TG explants (lanes 1 to 3), latently infected TG cells that reactivated during culture establishment or following heat stress (lanes 4 to 6), or latently infected TG cell cultures superinfected with ICP4− virus (lanes 7 to 9). The controls were total DNAs obtained from L7 cells infected with viral stocks of KOS, _n_212, or 7134.
Similar articles
- Optimized viral dose and transient immunosuppression enable herpes simplex virus ICP0-null mutants To establish wild-type levels of latency in vivo.
Halford WP, Schaffer PA. Halford WP, et al. J Virol. 2000 Jul;74(13):5957-67. doi: 10.1128/jvi.74.13.5957-5967.2000. J Virol. 2000. PMID: 10846077 Free PMC article. - ICP0, ICP4, or VP16 expressed from adenovirus vectors induces reactivation of latent herpes simplex virus type 1 in primary cultures of latently infected trigeminal ganglion cells.
Halford WP, Kemp CD, Isler JA, Davido DJ, Schaffer PA. Halford WP, et al. J Virol. 2001 Jul;75(13):6143-53. doi: 10.1128/JVI.75.13.6143-6153.2001. J Virol. 2001. PMID: 11390616 Free PMC article. - ICP0 is not required for efficient stress-induced reactivation of herpes simplex virus type 1 from cultured quiescently infected neuronal cells.
Miller CS, Danaher RJ, Jacob RJ. Miller CS, et al. J Virol. 2006 Apr;80(7):3360-8. doi: 10.1128/JVI.80.7.3360-3368.2006. J Virol. 2006. PMID: 16537603 Free PMC article. - A comparison of herpes simplex virus type 1 and varicella-zoster virus latency and reactivation.
Kennedy PG, Rovnak J, Badani H, Cohrs RJ. Kennedy PG, et al. J Gen Virol. 2015 Jul;96(Pt 7):1581-602. doi: 10.1099/vir.0.000128. Epub 2015 Mar 20. J Gen Virol. 2015. PMID: 25794504 Free PMC article. Review. - Human alpha-herpesvirus 1 (HSV-1) viral replication and reactivation from latency are expedited by the glucocorticoid receptor.
Jones C. Jones C. J Virol. 2025 Apr 15;99(4):e0030325. doi: 10.1128/jvi.00303-25. Epub 2025 Mar 27. J Virol. 2025. PMID: 40145740 Free PMC article. Review.
Cited by
- Centromere architecture breakdown induced by the viral E3 ubiquitin ligase ICP0 protein of herpes simplex virus type 1.
Gross S, Catez F, Masumoto H, Lomonte P. Gross S, et al. PLoS One. 2012;7(9):e44227. doi: 10.1371/journal.pone.0044227. Epub 2012 Sep 20. PLoS One. 2012. PMID: 23028505 Free PMC article. - The herpes simplex virus type 1 ICP0 promoter is activated by viral reactivation stimuli in trigeminal ganglia neurons of transgenic mice.
Loiacono CM, Taus NS, Mitchell WJ. Loiacono CM, et al. J Neurovirol. 2003 Jun;9(3):336-45. doi: 10.1080/13550280390201047. J Neurovirol. 2003. PMID: 12775417 - Stress Hormones Epinephrine and Corticosterone Selectively Reactivate HSV-1 and HSV-2 in Sympathetic and Sensory Neurons.
Goswami P, Ives AM, Abbott ARN, Bertke AS. Goswami P, et al. Viruses. 2022 May 23;14(5):1115. doi: 10.3390/v14051115. Viruses. 2022. PMID: 35632856 Free PMC article. - Herpes simplex virus 1 targets IRF7 via ICP0 to limit type I IFN induction.
Shahnazaryan D, Khalil R, Wynne C, Jefferies CA, Ní Gabhann-Dromgoole J, Murphy CC. Shahnazaryan D, et al. Sci Rep. 2020 Dec 17;10(1):22216. doi: 10.1038/s41598-020-77725-4. Sci Rep. 2020. PMID: 33335135 Free PMC article. - "Non-Essential" Proteins of HSV-1 with Essential Roles In Vivo: A Comprehensive Review.
Dogrammatzis C, Waisner H, Kalamvoki M. Dogrammatzis C, et al. Viruses. 2020 Dec 23;13(1):17. doi: 10.3390/v13010017. Viruses. 2020. PMID: 33374862 Free PMC article. Review.
References
- Clements G B, Stow N D. A herpes simplex virus type 1 mutant containing a deletion within immediate early gene 1 is latency-competent in mice. J Gen Virol. 1989;70:2501–2506. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F32 AI010147/AI/NIAID NIH HHS/United States
- P01 NS035138/NS/NINDS NIH HHS/United States
- F32 AI 10147/AI/NIAID NIH HHS/United States
- P01 NS 35138/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous