Phosphorylation and rapid relocalization of 53BP1 to nuclear foci upon DNA damage - PubMed (original) (raw)
Phosphorylation and rapid relocalization of 53BP1 to nuclear foci upon DNA damage
L Anderson et al. Mol Cell Biol. 2001 Mar.
Abstract
53BP1 is a human BRCT protein that was originally identified as a p53-interacting protein by the Saccharomyces cerevisiae two-hybrid screen. Although the carboxyl-terminal BRCT domain shows similarity to Crb2, a DNA damage checkpoint protein in fission yeast, there is no evidence so far that implicates 53BP1 in the checkpoint. We have identified a Xenopus homologue of 53BP1 (XL53BP1). XL53BP1 is associated with chromatin and, in some cells, localized to a few large foci under normal conditions. Gamma-ray irradiation induces increased numbers of the nuclear foci in a dose-dependent manner. The damage-induced 53BP1 foci appear rapidly (in 30 min) after irradiation, and de novo protein synthesis is not required for this response. In human cells, 53BP1 foci colocalize with Mrel1 foci at later stages of the postirradiation period. XL53BP1 is hyperphosphorylated after X-ray irradiation, and inhibitors of ATM-related kinases delay the relocalization and reduce the phosphorylation of XL53BP1 in response to X-irradiation. In AT cells, which lack ATM kinase, the irradiation-induced responses of 53BP1 are similarly affected. These results suggest a role for 53BP1 in the DNA damage response and/or checkpoint control which may involve signaling of damage to p53.
Figures
FIG. 1
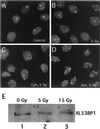
(A) Verification of antibodies against 53BP1 and immunoprecipitation. Cell lysates were prepared from Xenopus cells (XTC-2), (lanes 1 and 2) or from HeLa cells (lanes 3 and 4), run on an SDS-polyacrylamide gel, and subjected to immunoblotting. Affinity-purified anti-XL53BP1 (Xenopus cells; lane 1) or anti-HS53BP1 (human; lane 4) antibodies react with single proteins in the extracts. Preimmune sera were used as negative controls (lanes 2 and 3). Lane 6, fluorography of immunoprecipitate (IP) with anti-HS53BP1 antibody from 35S-labeled HeLa cell extracts lane 5, immunoprecipitation with preimmune serum control. (B) Gel filtration of HeLa cell extracts. The extract (Lo) was loaded on a Superdex 200 (10/30) column, and the fractions were analyzed by immunoblotting with anti-HS53BP1 antibodies. Positions where the molecular mass markers were eluted are indicated below the gel in kilodaltons.
FIG. 2
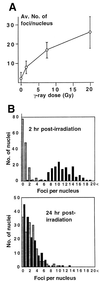
XL53BP1 localizes to nuclear foci, which increase in number after DNA damage. Xenopus XTC-2 cells were immunostained with anti-XL53BP1 antibodies. (A) In control cells, there were two types of XL53BP1 populations. One was homogeneously distributed over chromatin, and the other was associated with large foci. (B) Staining with preimmune serum showed very little background. (C to E) After exposure to γ rays, XL53BP1 formed increased numbers of smaller foci dispersed throughout the nucleus. γ-Ray dosages were 1.5 Gy (C), 7.5 Gy (D), and 20 Gy (E). (F to I) XL53BP1 foci were also induced by various DNA-damaging agents. Cells were subjected to 3.3 μg of VM26 per ml (F), 60 μg of mitomycin C per ml (G), 300 J of UV per m2 (H), and 10 mM hydroxyurea (HU) (I). Cells were fixed for staining at 2 h postirradiation or at 16 h after addition of the chemicals.
FIG. 3
(A) γ-Ray-dose-dependent increase of XL53BP1 focal number. The average number of foci per nucleus was plotted versus radiation dose. Error bars indicate standard deviations. (B) Recovery of the XL53BP1 focal phenotype after γ-ray irradiation. XTC-2 cells were either untreated (stippled bars) or irradiated with 5 Gy of γ rays (black bars) and fixed for XL53BP1 staining at 2 h or at 24 h postirradiation. Optical sections were recorded by confocal microscope. The number of XL53BP1 foci per nucleus was counted in 150 cells for each sample. At 2 h postirradiation, the nuclei had 10.9 ± 3.2 XL53BP1 foci (upper graph, black bars). At 24 h postirradiation, the average focus number per nucleus decreased to 1.99 ± 2.15 (lower graph, black bars), which was equivalent to that of the untreated control cells (stippled bars).
FIG. 4
Neither inhibitors of transcription nor translation blocks the assembly of XL53BP1. XTC-2 cells were irradiated with 5 Gy of γ rays in the presence of 50 μg of cycloheximide (Cyh) per ml (C) or 25 μg of actinomycin D (Act) per ml (D). (A) Nonirradiated control. (B) Cells irradiated without the inhibitors. (E) the amount of XL53BP1 did not increase after γ-ray irradiation. XTC-2 cells were exposed to 5 or 15 Gy of γ rays, and at 2 h after irradiation, cell extracts were prepared for immunoblotting with anti-XL53BP1 antibody. Each slot was loaded with the same amount of proteins.
FIG. 5
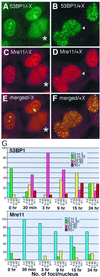
Colocalization of 53BP1 foci and Mre11 foci in human HT1080 cells. HT1080 cells untreated (A, C, and E) or exposed to 84 Gy of X rays (X) (B, D, and F) were fixed at 15 h postirradiation and double stained with Cy5 for Mre11 (C and D; pseudo-colored in red) and with FITC for 53BP1 (A and B; green). (E and F) Merged images of panels A and C and of B and D, respectively. Colocalization of green foci and red foci yields yellow signals. The cell marked by an arrowhead in panels B and D had 53BP1 foci but not discrete Mre11 foci. The cells marked by asterisks in panels C and D were in mitosis, and 53BP1 and Mre11 were dissociated from mitotic chromosomes and spread throughout the cytoplasm. (G) Kinetics of 53BP1 and Mre11 focal formation. HT1080 cells were either untreated (0 h) or irradiated with X rays, fixed at the indicated times postirradiation, and stained independently for either 53BP1 or Mre11. Optical sections were recorded by confocal microscope. The number of 53BP1 foci (upper graph) or Mre11 (lower graph) foci per nucleus was counted in 50 cells for each sample. Nuclei were categorized as having 0 to 5 foci (blue bars), 6 to 30 foci (green bars), 31 to 60 foci (yellow bars), 61 to 90 foci (red bars), and >91 foci (brown bars) at each time point. The y axis indicates the percentage of each category at each time point. For Mre11, only large, well-distinguished foci were scored (e.g., the nucleus marked with an arrowhead in panel D was scored as negative for Mre11 foci). Thus, the number of Mre11 foci may have been underestimated. Note that 53BP1 foci form early at 30 min postirradiation, when Mre11 foci were not observed at all.
FIG. 6
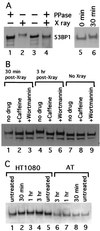
Phosphorylation of XL53BP1 in cells irradiated with X rays. (A) XL53BP1 was immunoprecipitated from XTC-2 cells either untreated (lanes 1 and 3; − X ray) or exposed to X rays (lanes 2 and 4; + X ray) at 2 h postirradiation. Half of the samples were treated with protein phosphatase (lanes 1 and 4; + PPase). The proteins were run on a gel and immunoblotted with anti-XL53BP1 antibodies. XL53BP1 of irradiated cells migrated more slowly than that of nonirradiated cells (lanes 2). This retardation was lost if the sample was phosphatased (lane 4). Lanes 5 and 6 indicate that the band retardation was already seen at 30 min postirradiation. (B) Caffeine blocks the phosphorylation of XL53BP1. XTC-2 cells were either untreated (lanes 7 to and 9) or exposed to X rays (lanes 1 to 6) in the absence of drugs (lanes 1, 4, and 7) or in the presence of 16 mM caffeine (lanes 2, 5, and 8) or 23 μM wortmannin (lanes 3, 6, and 9). Extracts were prepared from irradiated cells at 30 min (lanes 1, 2, and 3) and at 3 h (lanes 4 to 6) post-X-ray exposure. The extent of the band retardation was significantly reduced in the presence of caffeine (e.g., compare lanes 4 and 5). Wortmannin had a similar effect but with lower efficiency (lane 6). The same amount of protein was loaded per slot. The lower bands were degradation products of XL53BP1. (C) AT cells show a reduction in 53BP1 phosphorylation in response to X-ray irradiation. Extracts were prepared from untreated cells (lanes 1, 5, and 9) or from cells irradiated with X rays, at 30 min (lanes 2 and 8), at 1 h (lanes 3 and 7), and at 3 h (lanes 4 and 6) postirradiation. In control ATM+ HT1080 cells (lanes 1 to 5), 53BP1 showed slower migration after X-ray irradiation (lanes 2 to 4). In AT cells that are ATM kinase defective (lanes 6 to 9), the retardation was greatly reduced and the 53BP1 band showed only a slight retardation at 1 and 3 h compared with that of the untreated control (compare lanes 6 and 7 to 9). 53BP1 from the untreated HT1080 cells ran slightly more slowly than that from AT cells for unknown reasons. Samples were loaded in an order to see the shifted and the nonshifted bands next to each other.
FIG. 7
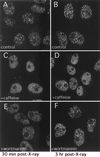
XL53BP1 focus formation is diminished or delayed in the presence of caffeine or wortmannin, respectively. Cells were treated as described for Fig. 6. XTC-2 cells were exposed to X rays either in the absence of any drugs (A and B) or in the presence of 16 mM caffeine (C and D) or 23 μM wortmannin (E and F) and fixed at 30 min (A, C, and E) or at 3 h (B, D, and F) postirradiation for XL53BP1 stain.
FIG. 8
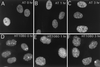
AT cells show a slight delay in 53BP1 focus formation in response to X-ray irradiation. AT cells (A to C) or HT1080 cells (D to F) were either untreated (A and D) or exposed to X rays, fixed at 1 h (B and E) or at 3 h (C and F) postirradiation, and stained for 53BP1.
Similar articles
- p53 binding protein 1 (53BP1) is an early participant in the cellular response to DNA double-strand breaks.
Schultz LB, Chehab NH, Malikzay A, Halazonetis TD. Schultz LB, et al. J Cell Biol. 2000 Dec 25;151(7):1381-90. doi: 10.1083/jcb.151.7.1381. J Cell Biol. 2000. PMID: 11134068 Free PMC article. - Tumor suppressor p53 binding protein 1 (53BP1) is involved in DNA damage-signaling pathways.
Rappold I, Iwabuchi K, Date T, Chen J. Rappold I, et al. J Cell Biol. 2001 Apr 30;153(3):613-20. doi: 10.1083/jcb.153.3.613. J Cell Biol. 2001. PMID: 11331310 Free PMC article. - Human PTIP facilitates ATM-mediated activation of p53 and promotes cellular resistance to ionizing radiation.
Jowsey PA, Doherty AJ, Rouse J. Jowsey PA, et al. J Biol Chem. 2004 Dec 31;279(53):55562-9. doi: 10.1074/jbc.M411021200. Epub 2004 Sep 27. J Biol Chem. 2004. PMID: 15456759 - ATM signaling and 53BP1.
Zgheib O, Huyen Y, DiTullio RA Jr, Snyder A, Venere M, Stavridi ES, Halazonetis TD. Zgheib O, et al. Radiother Oncol. 2005 Aug;76(2):119-22. doi: 10.1016/j.radonc.2005.06.026. Radiother Oncol. 2005. PMID: 16024119 Review. - Checkpoints: it takes more than time to heal some wounds.
Rhind N, Russell P. Rhind N, et al. Curr Biol. 2000 Dec 14-28;10(24):R908-11. doi: 10.1016/s0960-9822(00)00849-6. Curr Biol. 2000. PMID: 11137027 Free PMC article. Review.
Cited by
- Expression pattern of p53-binding protein 1 as a new molecular indicator of genomic instability in bladder urothelial carcinoma.
Matsuda K, Kawasaki T, Akazawa Y, Hasegawa Y, Kondo H, Suzuki K, Iseki M, Nakashima M. Matsuda K, et al. Sci Rep. 2018 Oct 19;8(1):15477. doi: 10.1038/s41598-018-33761-9. Sci Rep. 2018. PMID: 30341375 Free PMC article. - Spatiotemporal characterization of ionizing radiation induced DNA damage foci and their relation to chromatin organization.
Costes SV, Chiolo I, Pluth JM, Barcellos-Hoff MH, Jakob B. Costes SV, et al. Mutat Res. 2010 Apr-Jun;704(1-3):78-87. doi: 10.1016/j.mrrev.2009.12.006. Epub 2010 Jan 8. Mutat Res. 2010. PMID: 20060491 Free PMC article. Review. - Regulation of TIP60 by ATF2 modulates ATM activation.
Bhoumik A, Singha N, O'Connell MJ, Ronai ZA. Bhoumik A, et al. J Biol Chem. 2008 Jun 20;283(25):17605-14. doi: 10.1074/jbc.M802030200. Epub 2008 Apr 8. J Biol Chem. 2008. PMID: 18397884 Free PMC article. - The FHA and BRCT domains recognize ADP-ribosylation during DNA damage response.
Li M, Lu LY, Yang CY, Wang S, Yu X. Li M, et al. Genes Dev. 2013 Aug 15;27(16):1752-68. doi: 10.1101/gad.226357.113. Genes Dev. 2013. PMID: 23964092 Free PMC article.
References
- Blasina A, Price B D, Turenne G A, McGowan C H. Caffeine inhibits the checkpoint kinase ATM. Curr Biol. 1999;9:1135–1138. - PubMed
- Bork P, Hofmann K, Bucher P, Neuwald A F, Altschul S F, Koonin E V. A superfamily of conserved domains in DNA damage-responsive cell cycle checkpoint proteins. FASEB J. 1997;11:68–76. - PubMed
- Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
- Carr A M. Piecing together the p53 puzzle. Science. 2000;287:1765–1766. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous