Delivery of proteins into living cells by reversible membrane permeabilization with streptolysin-O - PubMed (original) (raw)
Delivery of proteins into living cells by reversible membrane permeabilization with streptolysin-O
I Walev et al. Proc Natl Acad Sci U S A. 2001.
Abstract
The pore-forming toxin streptolysin O (SLO) can be used to reversibly permeabilize adherent and nonadherent cells, allowing delivery of molecules with up to 100 kDa mass to the cytosol. Using FITC-labeled albumin, 10(5)-10(6) molecules were estimated to be entrapped per cell. Repair of toxin lesions depended on Ca(2+)-calmodulin and on intact microtubules, but was not sensitive to actin disruption or to inhibition of protein synthesis. Resealed cells were viable for days and retained the capacity to endocytose and to proliferate. The active domains of large clostridial toxins were introduced into three different cell lines. The domains were derived from Clostridium difficile B-toxin and Clostridium sordelli lethal toxin, which glycosylate small G-proteins, and from Clostridium botulinum C2 toxin, which ADP-ribosylates actin. After delivery with SLO, all three toxins disrupted the actin cytoskeleton to cause rounding up of the cells. Glucosylation assays demonstrated that G-proteins Rho and Ras were retained in the permeabilized cells and were modified by the respective toxins. Inactivation of these G-proteins resulted in reduced stimulus-dependent granule secretion, whereas ADP-ribosylation of actin by the C. botulinum C2-toxin resulted in enhanced secretion in cells. The presented method for introducing proteins into living cells should find multifaceted application in cell biology.
Figures
Figure 1
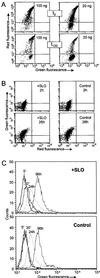
(A) Demonstration of membrane resealing by flow cytometric analysis of propidium iodide influx into cells. THP cells were incubated with SLO at the given concentrations. Addition of propidium iodide after 10 min resulted in red fluorescence of permeabilized cells. After repair (t120), the majority of cells treated with 20 ng/ml SLO became impermeable to the dye. (B) Proliferation assay. Flow cytometric analysis of 5-brom-2′-desoxyuridine incorporation performed after 2 h and 26 h following resealing revealed no difference to the controls. (C) Endocytosis assay. No alterations in endocytic uptake of FITC-dextran were observed by flow cytometry between toxin-treated and control cells.
Figure 2
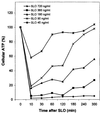
Recovery of cellular ATP levels following SLO-treatment. Adherent COS-cells were permeabilized with SLO at the depicted concentrations for 10 min, after which resealing was initiated. Total cellular ATP was quantified at the given times. The experiment was reproduced two times with similar results.
Figure 3
Entrapment of FITC-dextrans of varying size. THP cells were permeabilized with 20 ng/ml SLO in the presence of FITC-dextrans of the given masses, and propidium iodide was added after the resealing period. Panels on the right show the phase contrast photographs, and the corresponding fluorescence microscopic pictures are depicted on the left. The molecular weight cutoff for dextrans that could be delivered was in the range of 150 kDa.
Figure 4
Glucosylation of RBL 2H3 lysates. RBL 2H3 cells were permeabilized with SLO (100 ng/ml) alone or in the presence or absence of active or heat-inactivated CS546 (3 μg/ml) for 10 min. Controls were left unpermeabilized in buffer, with or without active CD546 (3 μg/ml). After resealing, the cells were washed and incubated for 2 h. Cells were then scraped off in the presence of lysis buffer and sonicated five times for 5 s on ice. Thereafter, the lysates (1 mg/ml) were incubated with 1 μg/ml CS546 and 20 μM UDP[14C] glucose for 30 min at 37°C. Labeled proteins were analyzed by SDS/PAGE. PhosphorImager data from SDS/PAGE are shown.
Figure 5
Morphological changes of RBL 2H3 cells upon treatment with SLO, CDB546, or CS546. Cells were incubated with SLO (100 ng/ml) alone for 10 min (A), or in combination with either 3 μg/ml CDB546 (B) or 3 μg/ml CS546 (C). Pictures were taken after 90 min resealing.
Figure 6
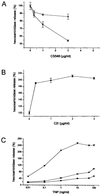
(A) Decreased hexosaminidase release in SLO permeabilized RBL 2H3 cells after treatment with CS546 or CDB546. RBL cells were exposed to 100 ng/ml (■) or 50 ng/ml (▴) SLO in the presence of CD546 (A) or CDB546 (B) in the depicted concentrations. After 2 h resealing, cells were stimulated with TNP (50 ng/ml) and hexosaminidase release was measured. Hexosaminidase release of cells treated with SLO only was set at 100%. (B) Increased hexosaminidase release in SLO permeabilized RBL 2H3 cells after treatment with C2I. RBL cells were exposed to 100 ng/ml SLO in the presence of C2I in the depicted concentrations. After 2 h resealing, cells were stimulated with TNP (50 ng/ml). Secretion in control cells was set as 100%. (C) Hexosaminidase release in SLO permeabilized RBL 2H3 cells after treatment with C2I or CS546. RBL cells were exposed to 100 ng/ml SLO alone (●) or in the presence of C2I (2 μg/ml, ▴) or CS546 (5 μg/ml, ■). After 2 h resealing, cells were stimulated with TNP in the depicted concentrations and hexosaminidase release was measured.
Similar articles
- [Analysis of synaptic neurotransmitter release mechanisms using bacterial toxins].
Doussau F, Humeau Y, Vitiello F, Popoff MR, Poulain B. Doussau F, et al. J Soc Biol. 1999;193(6):457-67. J Soc Biol. 1999. PMID: 10783704 Review. French. - Inhibition of insulin-stimulated glucose transport in 3T3-L1 cells by Clostridium difficile toxin B, Clostridium sordellii lethal toxin, and Clostridium botulinum C2 toxin.
Schmid G, Schürmann A, Huppertz C, Hofmann F, Aktories K, Joost HG. Schmid G, et al. Naunyn Schmiedebergs Arch Pharmacol. 1998 Apr;357(4):385-92. doi: 10.1007/pl00005183. Naunyn Schmiedebergs Arch Pharmacol. 1998. PMID: 9606023 - Observation of NMR signals from proteins introduced into living mammalian cells by reversible membrane permeabilization using a pore-forming toxin, streptolysin O.
Ogino S, Kubo S, Umemoto R, Huang S, Nishida N, Shimada I. Ogino S, et al. J Am Chem Soc. 2009 Aug 12;131(31):10834-5. doi: 10.1021/ja904407w. J Am Chem Soc. 2009. PMID: 19603816 - G-protein-stimulated phospholipase D activity is inhibited by lethal toxin from Clostridium sordellii in HL-60 cells.
El Hadj NB, Popoff MR, Marvaud JC, Payrastre B, Boquet P, Geny B. El Hadj NB, et al. J Biol Chem. 1999 May 14;274(20):14021-31. doi: 10.1074/jbc.274.20.14021. J Biol Chem. 1999. PMID: 10318815 - Clostridium difficile Toxin Biology.
Aktories K, Schwan C, Jank T. Aktories K, et al. Annu Rev Microbiol. 2017 Sep 8;71:281-307. doi: 10.1146/annurev-micro-090816-093458. Epub 2017 Jun 28. Annu Rev Microbiol. 2017. PMID: 28657883 Review.
Cited by
- Multiwell-based G0-PCC assay for radiation biodosimetry.
Royba E, Shuryak I, Ponnaiya B, Repin M, Pampou S, Karan C, Turner H, Garty G, Brenner DJ. Royba E, et al. Sci Rep. 2024 Aug 26;14(1):19789. doi: 10.1038/s41598-024-69243-4. Sci Rep. 2024. PMID: 39187542 Free PMC article. - NADPH oxidase is internalized by clathrin-coated pits and localizes to a Rab27A/B GTPase-regulated secretory compartment in activated macrophages.
Ejlerskov P, Christensen DP, Beyaie D, Burritt JB, Paclet MH, Gorlach A, van Deurs B, Vilhardt F. Ejlerskov P, et al. J Biol Chem. 2012 Feb 10;287(7):4835-52. doi: 10.1074/jbc.M111.293696. Epub 2011 Dec 8. J Biol Chem. 2012. PMID: 22157766 Free PMC article. - RNA structure promotes liquid-to-solid phase transition of short RNAs in neuronal dysfunction.
Wang S, Xu Y. Wang S, et al. Commun Biol. 2024 Jan 29;7(1):137. doi: 10.1038/s42003-024-05828-z. Commun Biol. 2024. PMID: 38287096 Free PMC article. - Anthrolysin O and fermentation products mediate the toxicity of Bacillus anthracis to lung epithelial cells under microaerobic conditions.
Popova TG, Millis B, Chung MC, Bailey C, Popov SG. Popova TG, et al. FEMS Immunol Med Microbiol. 2011 Feb;61(1):15-27. doi: 10.1111/j.1574-695X.2010.00740.x. Epub 2010 Oct 14. FEMS Immunol Med Microbiol. 2011. PMID: 20946354 Free PMC article. - Sensitivities of human monocytes and epithelial cells to pneumolysin are different.
Hirst RA, Yesilkaya H, Clitheroe E, Rutman A, Dufty N, Mitchell TJ, O'Callaghan C, Andrew PW. Hirst RA, et al. Infect Immun. 2002 Feb;70(2):1017-22. doi: 10.1128/IAI.70.2.1017-1022.2002. Infect Immun. 2002. PMID: 11796644 Free PMC article.
References
- Wilson A K, Horwitz J, de Lanerolle P. Am J Physiol. 1991;260:C355–C363. - PubMed
- Lauer J L, Fields G B. Methods Enzymol. 1997;289:564–571. - PubMed
- Derossi D, Chassaing G, Prochiantz A. Trends Cell Biol. 1998;8:84–87. - PubMed
- Bhakdi S, Bayley H, Valeva A, Walev I, Walker B, Kehoe M, Palmer M. Arch Microbiol. 1996;165:73–79. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous