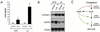The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity - PubMed (original) (raw)
. 2001 Mar 13;98(6):3369-74.
doi: 10.1073/pnas.051551698.
B Goodwin, S A Jones, D Hawkins-Brown, K I MacKenzie, A LaTour, Y Liu, C D Klaassen, K K Brown, J Reinhard, T M Willson, B H Koller, S A Kliewer
Affiliations
- PMID: 11248085
- PMCID: PMC30660
- DOI: 10.1073/pnas.051551698
The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity
J L Staudinger et al. Proc Natl Acad Sci U S A. 2001.
Abstract
The pregnane X receptor (PXR) is the molecular target for catatoxic steroids such as pregnenolone 16alpha-carbonitrile (PCN), which induce cytochrome P450 3A (CYP3A) expression and protect the body from harmful chemicals. In this study, we demonstrate that PXR is activated by the toxic bile acid lithocholic acid (LCA) and its 3-keto metabolite. Furthermore, we show that PXR regulates the expression of genes involved in the biosynthesis, transport, and metabolism of bile acids including cholesterol 7alpha-hydroxylase (Cyp7a1) and the Na(+)-independent organic anion transporter 2 (Oatp2). Finally, we demonstrate that activation of PXR protects against severe liver damage induced by LCA. Based on these data, we propose that PXR serves as a physiological sensor of LCA, and coordinately regulates gene expression to reduce the concentrations of this toxic bile acid. These findings suggest that PXR agonists may prove useful in the treatment of human cholestatic liver disease.
Figures
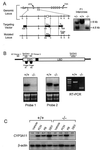
Figure 1
Disruption of the PXR gene in ES cells and mice. (A) Schematic representation of the PXR locus segment, the targeting vector and the targeted PXR allele. Open boxes indicate exons and labeled boxes indicate the PGK-tk and PGK-neo cassettes. Selected restriction endonuclease sites are indicated: B, _Bam_HI; K,_Kpn_I; N, _Nhe_I; S, _Spe_I; and X, _Xho_I. A novel _Bam_HI site is introduced into the PXR locus by the homologous recombination event allowing the targeted locus to be distinguished from the wild-type allele by Southern analysis of _Bam_HI digested DNA with the indicated probe (shown at the right). (B) Northern blot analysis of RNA isolated from the livers of wild-type and PXR−/− mice by using the probes indicated in the schematic of the PXR cDNA. Reverse transcription (RT)–PCR was performed using the primers indicated in the schematic. Amplification of the wild-type and disrupted PXR mRNAs yielded products of 111 bp and 363 bp, respectively. (C) Total RNA was prepared from the livers of three wild-type and PXR−/− mice treated with PCN, PB, dexamethasone, or vehicle alone. RNA samples were pooled before Northern blot analysis with probes for Cyp3a11. Bands were quantitated as described in Materials and Methods and represent the mean obtained from three animals in each treatment group. Values are normalized to β-actin and are expressed as fold change relative to wild-type mice receiving vehicle alone.
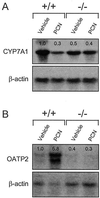
Figure 2
PXR represses Cyp7a1 and induces Oatp2 expression in response to PCN. Total RNA was prepared from the livers of PXR+/+ and PXR−/− mice treated with PCN or vehicle alone. RNA from three animals was pooled and subjected to Northern blot analysis with probes for (A) Cyp7a1, (B) Oatp2, and β-actin. Quantitation of bands was performed as described in Fig. 1.
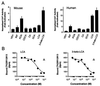
Figure 3
Bile acids bind and activate PXR. (A) CV-1 cells were transfected with expression plasmids for mouse PXR or human PXR and the reporter plasmid (Cyp3a23)2-tk-CAT, and the cells treated with 100 μM concentrations of the indicated bile acids: CDCA, chenodeoxycholic acid; CA, cholic acid; DCA, deoxycholic acid; LCA, lithocholic acid; 3-keto-LCA, 3-keto-lithocholic acid. PCN and rifampicin (RIF) were used as positive controls for mPXR and hPXR, respectively, at 10 μM concentration. Cell extracts were subsequently assayed for CAT activity. Data represent the mean ± SD of assays performed in triplicate. Activation of mPXR by LCA is highly statistically significant as measured by the Student's_t_ test (P < 0.001). (B) Scintillation proximity competition binding assays were performed with human PXR ligand binding domain and 10 nM [3H]SR12813 in the presence of increasing concentrations of LCA and 3-keto-LCA. A single-point negative control (tauro-β-muricholic acid, open triangle) is also shown.
Figure 4
Effects of treating wild-type and PXR−/− mice with LCA. (A) Wild-type and PXR−/− mice were fed a diet supplemented with 0.5% LCA for 8 days. Urine was collected on day 8 of the study and LCA concentrations were determined. Data represent the mean ± SEM of assays performed on urine from eight different mice. ND, not detected; *, Statistically significantly difference between wild-type and PXR−/−,P < 0.05. (B) Total RNA was isolated and pooled from the livers of six wild-type and PXR−/− mice injected for 4 days with LCA or vehicle alone and subject to Northern blot analysis with probes for Cyp3a11, Oatp2, and β-actin. (C) Model for PXR as a bile acid sensor. Activation of PXR by LCA and/or LCA metabolites results in repression of Cyp7a1, which blocks bile acid biosynthesis, and induction of Oatp2 and Cyp3a expression, which promote bile acid uptake and metabolism. The net effect is to prevent the accumulation of bile acids to toxic levels.
Figure 5
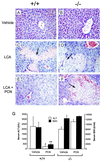
PXR activation protects against LCA-induced hepatotoxicity. Wild-type (A, C, and E) and PXR−/− (B, D, and_E_) mice were treated with vehicle alone (A and B), LCA (C and_D_), or LCA and PCN (E and_F_). Liver sections were prepared for histology and stained with hematoxylin and eosin. Areas of severe necrosis in panels_C_, D, and E are indicated with arrows. (G) Serum ALT (open bars) and SDH (closed bars) levels were measured in wild-type and PXR−/− mice treated with either LCA and vehicle (Vehicle) or LCA and PCN (PCN). ALT and SDH values in untreated animals were 28 ± 10 units/l and 150 ± 20 units/ml, respectively. Data represent the mean ± SEM of assays performed with serum from six different animals. Statistical differences compared with the same genotype treated with LCA and vehicle: *, P < 0.005; **, P < 0.001.
Similar articles
- Coordinate regulation of xenobiotic and bile acid homeostasis by pregnane X receptor.
Staudinger J, Liu Y, Madan A, Habeebu S, Klaassen CD. Staudinger J, et al. Drug Metab Dispos. 2001 Nov;29(11):1467-72. Drug Metab Dispos. 2001. PMID: 11602523 - Pregnane X receptor mediated-transcription regulation of CYP3A by glycyrrhizin: a possible mechanism for its hepatoprotective property against lithocholic acid-induced injury.
Wang YG, Zhou JM, Ma ZC, Li H, Liang QD, Tan HL, Xiao CR, Zhang BL, Gao Y. Wang YG, et al. Chem Biol Interact. 2012 Oct 25;200(1):11-20. doi: 10.1016/j.cbi.2012.08.023. Epub 2012 Sep 13. Chem Biol Interact. 2012. PMID: 22982774 - The involvement of the pregnane X receptor in hepatic gene regulation during inflammation in mice.
Teng S, Piquette-Miller M. Teng S, et al. J Pharmacol Exp Ther. 2005 Feb;312(2):841-8. doi: 10.1124/jpet.104.076141. Epub 2004 Sep 29. J Pharmacol Exp Ther. 2005. PMID: 15456840 - Regulation of cyp3a gene transcription by the pregnane x receptor.
Goodwin B, Redinbo MR, Kliewer SA. Goodwin B, et al. Annu Rev Pharmacol Toxicol. 2002;42:1-23. doi: 10.1146/annurev.pharmtox.42.111901.111051. Annu Rev Pharmacol Toxicol. 2002. PMID: 11807162 Review. - Pregnane X receptor: molecular basis for species differences in CYP3A induction by xenobiotics.
LeCluyse EL. LeCluyse EL. Chem Biol Interact. 2001 May 16;134(3):283-9. doi: 10.1016/s0009-2797(01)00163-6. Chem Biol Interact. 2001. PMID: 11336976 Review.
Cited by
- Transcriptional Regulation of Cytosolic Sulfotransferase 1C2 by Vitamin D Receptor in LS180 Human Colorectal Adenocarcinoma Cells.
Barrett KG, Fang H, Kocarek TA, Runge-Morris M. Barrett KG, et al. Drug Metab Dispos. 2016 Aug;44(8):1431-4. doi: 10.1124/dmd.116.070300. Epub 2016 Apr 29. Drug Metab Dispos. 2016. PMID: 27130351 Free PMC article. - Critical roles of bile acids in regulating intestinal mucosal immune responses.
Sun R, Xu C, Feng B, Gao X, Liu Z. Sun R, et al. Therap Adv Gastroenterol. 2021 May 28;14:17562848211018098. doi: 10.1177/17562848211018098. eCollection 2021. Therap Adv Gastroenterol. 2021. PMID: 34104213 Free PMC article. Review. - Microbial Hydroxysteroid Dehydrogenases: From Alpha to Omega.
Doden HL, Ridlon JM. Doden HL, et al. Microorganisms. 2021 Feb 24;9(3):469. doi: 10.3390/microorganisms9030469. Microorganisms. 2021. PMID: 33668351 Free PMC article. Review. - Opposing regulation of cytochrome P450 expression by CAR and PXR in hypothyroid mice.
Park YJ, Lee EK, Lee YK, Park DJ, Jang HC, Moore DD. Park YJ, et al. Toxicol Appl Pharmacol. 2012 Sep 1;263(2):131-7. doi: 10.1016/j.taap.2012.03.017. Epub 2012 Apr 3. Toxicol Appl Pharmacol. 2012. PMID: 22503787 Free PMC article. - Bile acids regulation of cellular stress responses in liver physiology and diseases.
Li T, Hasan MN, Gu L. Li T, et al. eGastroenterology. 2024 Apr;2(2):e100074. doi: 10.1136/egastro-2024-100074. Epub 2024 May 31. eGastroenterology. 2024. PMID: 39027418 Free PMC article.
References
- Selye H. J Pharm Sci. 1971;60:1–28. - PubMed
- Kourounakis P, Selye H, Tache Y. Adv Steroid Biochem Pharmacol. 1977;6:35–57. - PubMed
- Guzelian P S. In: Microsomes and Drug Oxidations. Miners J O, Birkett D J, Drew R, McManus M, editors. London: Taylor and Francis; 1988. pp. 148–155.
- Maurel P. In: Cytochromes P 450, metabolic and toxicological aspects. Ioannides C, editor. Boca Raton, FL: CRC; 1996. pp. 241–270.
- Kliewer S A, Moore J T, Wade L, Staudinger J L, Watson M A, Jones S A, McKee D D, Oliver B B, Willson T M, Zetterstrom R H, et al. Cell. 1998;92:73–82. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials