CA1-specific N-methyl-D-aspartate receptor knockout mice are deficient in solving a nonspatial transverse patterning task - PubMed (original) (raw)
CA1-specific N-methyl-D-aspartate receptor knockout mice are deficient in solving a nonspatial transverse patterning task
L Rondi-Reig et al. Proc Natl Acad Sci U S A. 2001.
Abstract
In both humans and animals, the hippocampus is critical to memory across modalities of information (e.g., spatial and nonspatial memory) and plays a critical role in the organization and flexible expression of memories. Recent studies have advanced our understanding of cellular basis of hippocampal function, showing that N-methyl-d-aspartate (NMDA) receptors in area CA1 are required in both the spatial and nonspatial domains of learning. Here we examined whether CA1 NMDA receptors are specifically required for the acquisition and flexible expression of nonspatial memory. Mice lacking CA1 NMDA receptors were impaired in solving a transverse patterning problem that required the simultaneous acquisition of three overlapping odor discriminations, and their impairment was related to an abnormal strategy by which they failed to adequately sample and compare the critical odor stimuli. By contrast, they performed normally, and used normal stimulus sampling strategies, in the concurrent learning of three nonoverlapping concurrent odor discriminations. These results suggest that CA1 NMDA receptors play a crucial role in the encoding and flexible expression of stimulus relations in nonspatial memory.
Figures
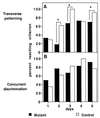
Figure 1
Percent of subjects reaching the performance criterion (eight of nine correct choices) on each training session in (A) transverse patterning and (B) concurrent discrimination. *, χ2; P < 0.001.
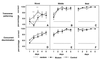
Figure 2
Mean percent correct responses on each training session for odor pairs associated with worst (A and D), middle (B and E), and best (C and_F_) accurate performance. (A_–_C) The transverse patterning problem. (D_–_F) The concurrent discrimination problem. *, Significantly different from chance; ANOVA;P < 0.05. **, Significantly different from both chance level and the score for CA1-NR1 mice; ANOVA;P < 0.001.
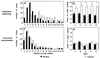
Figure 3
Distribution of the number of odor cups visited per trial combined across all sessions of (A) transverse patterning and (C) concurrent discrimination, and the mean number of odor cups visited on individual training sessions in (B) transverse patterning and (D) concurrent discrimination. *, ANOVA, P < 0.007.
Similar articles
- The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory.
Tsien JZ, Huerta PT, Tonegawa S. Tsien JZ, et al. Cell. 1996 Dec 27;87(7):1327-38. doi: 10.1016/s0092-8674(00)81827-9. Cell. 1996. PMID: 8980238 - Impaired sequential egocentric and allocentric memories in forebrain-specific-NMDA receptor knock-out mice during a new task dissociating strategies of navigation.
Rondi-Reig L, Petit GH, Tobin C, Tonegawa S, Mariani J, Berthoz A. Rondi-Reig L, et al. J Neurosci. 2006 Apr 12;26(15):4071-81. doi: 10.1523/JNEUROSCI.3408-05.2006. J Neurosci. 2006. PMID: 16611824 Free PMC article. - Enrichment induces structural changes and recovery from nonspatial memory deficits in CA1 NMDAR1-knockout mice.
Rampon C, Tang YP, Goodhouse J, Shimizu E, Kyin M, Tsien JZ. Rampon C, et al. Nat Neurosci. 2000 Mar;3(3):238-44. doi: 10.1038/72945. Nat Neurosci. 2000. PMID: 10700255 - Plasticity, hippocampal place cells, and cognitive maps.
Shapiro M. Shapiro M. Arch Neurol. 2001 Jun;58(6):874-81. doi: 10.1001/archneur.58.6.874. Arch Neurol. 2001. PMID: 11405801 Review. - The drugs don't work-or do they? Pharmacological and transgenic studies of the contribution of NMDA and GluR-A-containing AMPA receptors to hippocampal-dependent memory.
Bannerman DM, Rawlins JN, Good MA. Bannerman DM, et al. Psychopharmacology (Berl). 2006 Nov;188(4):552-66. doi: 10.1007/s00213-006-0403-6. Epub 2006 May 5. Psychopharmacology (Berl). 2006. PMID: 16676163 Review.
Cited by
- Dystrophin induced cognitive impairment: mechanisms, models and therapeutic strategies.
Anand A, Tyagi R, Mohanty M, Goyal M, Silva KR, Wijekoon N. Anand A, et al. Ann Neurosci. 2015 Apr;22(2):108-18. doi: 10.5214/ans.0972.7531.221210. Ann Neurosci. 2015. PMID: 26130916 Free PMC article. Review. - The hippocampus encodes delay and value information during delay-discounting decision making.
Masuda A, Sano C, Zhang Q, Goto H, McHugh TJ, Fujisawa S, Itohara S. Masuda A, et al. Elife. 2020 Feb 20;9:e52466. doi: 10.7554/eLife.52466. Elife. 2020. PMID: 32077851 Free PMC article. - The mechanisms for pattern completion and pattern separation in the hippocampus.
Rolls ET. Rolls ET. Front Syst Neurosci. 2013 Oct 30;7:74. doi: 10.3389/fnsys.2013.00074. Front Syst Neurosci. 2013. PMID: 24198767 Free PMC article. Review. - The hippocampus contributes to memory expression during transitive inference in mice.
Devito LM, Kanter BR, Eichenbaum H. Devito LM, et al. Hippocampus. 2010 Jan;20(1):208-17. doi: 10.1002/hipo.20610. Hippocampus. 2010. PMID: 19405137 Free PMC article. - Neurological disorders and therapeutics targeted to surmount the blood-brain barrier.
Kanwar JR, Sriramoju B, Kanwar RK. Kanwar JR, et al. Int J Nanomedicine. 2012;7:3259-78. doi: 10.2147/IJN.S30919. Epub 2012 Jul 9. Int J Nanomedicine. 2012. PMID: 22848160 Free PMC article. Review.
References
- Reed J, Squire L. Behav Neurosci. 1999;113:3–9. - PubMed
- Vargha-Khadem F, Gadian D G, Watkins K E, Connelly A, Van Paesschen W, Mishkin M. Science. 1997;277:376–380. - PubMed
- Teng E, Squire L R. Nature (London) 1999;12:675–677. - PubMed
- O'Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford: Clarendon; 1978.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous