Single point mutations affect fatty acid block of human myocardial sodium channel alpha subunit Na+ channels - PubMed (original) (raw)
Single point mutations affect fatty acid block of human myocardial sodium channel alpha subunit Na+ channels
Y F Xiao et al. Proc Natl Acad Sci U S A. 2001.
Abstract
Suppression of cardiac voltage-gated Na(+) currents is probably one of the important factors for the cardioprotective effects of the n-3 polyunsaturated fatty acids (PUFAs) against lethal arrhythmias. The alpha subunit of the human cardiac Na(+) channel (hH1(alpha)) and its mutants were expressed in human embryonic kidney (HEK293t) cells. The effects of single amino acid point mutations on fatty acid-induced inhibition of the hH1(alpha) Na(+) current (I(Na)) were assessed. Eicosapentaenoic acid (EPA, C20:5n-3) significantly reduced I(Na) in HEK293t cells expressing the wild type, Y1767K, and F1760K of hH1(alpha) Na(+) channels. The inhibition was voltage and concentration-dependent with a significant hyperpolarizing shift of the steady state of I(Na). In contrast, the mutant N406K was significantly less sensitive to the inhibitory effect of EPA. The values of the shift at 1, 5, and 10 microM EPA were significantly smaller for N406K than for the wild type. Coexpression of the beta(1) subunit and N406K further decreased the inhibitory effects of EPA on I(Na) in HEK293t cells. In addition, EPA produced a smaller hyperpolarizing shift of the V(1/2) of the steady-state inactivation in HEK293t cells coexpressing the beta(1) subunit and N406K. These results demonstrate that substitution of asparagine with lysine at the site of 406 in the domain-1-segment-6 region (D1-S6) significantly decreased the inhibitory effect of PUFAs on I(Na), and coexpression with beta(1) decreased this effect even more. Therefore, asparagine at the 406 site in hH1(alpha) may be important for the inhibition by the PUFAs of cardiac voltage-gated Na(+) currents, which play a significant role in the antiarrhythmic actions of PUFAs.
Figures
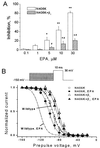
Figure 1
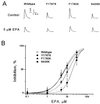
Concentration-dependent suppression of human cardiac Na+ currents by EPA. (A) Original current traces of INa in the absence (Control) and presence (EPA) of 5 μM EPA were evoked by depolarizing pulses from a holding potential of −150 mV to 30 mV for the wild type and three mutants. The membrane holding potential was −150 mV and the rate of pulses was 0.1 Hz. (B) The amplitude of peak Na+ currents shown as in A was calculated in the absence and presence of various concentrations of EPA. The EPA-induced inhibition of INa is concentration-dependent. Each data point represents the average value of at least seven individual cells.
Figure 2
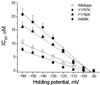
Effects of different holding potentials on the IC50 of EPA. INa was elicited by 10-ms test pulses from different holding potentials to 30 mV every 10 s. Concentration-dependent inhibitions of INa by EPA were fit with a logistical equation at different holding potentials, and IC50 of EPA was calculated by the equation. The data of IC50 were fit with the equation of linear regression for the wild type (○), Y1767K (●), F1760K (▴), and N406K (■) of hH1α Na+ channels. The regression coefficients were similar for the wild type (−0.157 ± 0.008) and Y1767K (−0.124 ± 0.015). However, the regression coefficients were significantly different (P < 0.01) between the wild type and N406K (−0.322 ± 0.018), as well as between the wild type and F1760K (−0.254 ± 0.014).
Figure 3
Voltage-dependent inhibition of INa by EPA in HEK293t cells expressing the wild type or three mutants of hH1α Na+ channels. (A) Whole-cell currents were normalized by INa recorded with the conditioning voltage of −180 mV for their corresponding controls. The experimental protocol is shown in the Inset. Currents were elicited by 10-ms test pulses to 30 mV following a 10-s conditioning pulse varying from −180 mV to −50 mV with 10-mV increments. A 100-ms interval was inserted between the conditioning pulse and the test pulse. The membrane potential was held at −150 mV, and the pulse rate was 0.1 Hz. Except for the mutant F1760K, EPA at 5 μM did not show profound voltage-dependent suppression of INa for the wild type, Y1767K, and N406K with the protocol of a 100-ms recovery interval. (B) Voltage-dependent suppression of INa in the presence of 5 μM EPA is shown. The Inset is the voltage protocol with a recovery interval of 5 ms. EPA at 5 μM produced a profound voltage-dependent suppression of INa for the wild type, Y1767K, and F1760K, but the suppression for N406K is relatively less profound with the protocol of a 5-ms recovery interval. The data were fit with a Boltzmann equation.
Figure 4
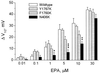
Effects of EPA on the shift of the steady-state inactivation of INa. Currents were elicited by 10-ms test pulses to 30 mV following 500-ms conditional prepulses varying from −160 mV to −20 mV with 10-mV increments. The experimental protocol was the same as shown for the Inset of Fig. 6_B_. The membrane potential was held at −150 mV, and the pulse rate was 0.1 Hz. Normalized steady-state inactivation was averaged and fit with a Boltzmann equation. The values of V1/2 voltages were calculated in the absence and presence of EPA. Delta changes of hyperpolarizing shift of V1/2 caused by various concentrations of EPA were plotted for the wild type, Y1767K, F1760K, and N406K of hH1α Na+ channels. Each bar represents the mean and SE of at least eight individual cells. *,P < 0.05; **, P < 0.01; ***,P < 0.001; N406K versus the wild type or the other two mutants, Y1767K and F1760K.
Figure 5
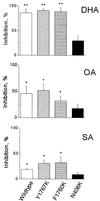
Inhibition of INa by other fatty acids. Na+ currents were evoked by single-step voltage pulses from a holding potential of −120 to 30 mV. INa of the wild type, Y1767K, and F1760K was significantly inhibited by 5 μM docosahexaenoic acid (DHA, C22:6n-3), oleic acid (OA, C18:1n-9), or stearic acid (SA, C18:0). In contrast, INa of N406K was not significantly inhibited by the fatty acids. Each bar represents the average value of at least four individual cells. *, P < 0.05; **,P < 0.01; INa in the presence of 5 μM fatty acids versus the corresponding control for the wild type or their mutants.
Figure 6
Effects of coexpressing β1 subunits on the steady-state inactivation and EPA-induced inhibition of Na+ currents in HEK293t cells expressing N406K or plus the β1 subunit. (A) Concentration-dependent inhibition of Na+ currents by EPA in HEK293t cells transfected with N406K alone (N406K) or cotransfected with N406K and β1 subunits (N406K + β1). Each bar represents the average value of at least seven individual cells. *, P < 0.05; **,P < 0.01; versus control. #, P < 0.05; N406K plus β1 versus N406K. (B) The effects of coexpressing β1 subunits on the steady-state inactivation of INa in the absence or presence of 5 μM EPA for N406K and N406K plus β1. Currents were elicited by 10-ms test pulses to 30 mV following 500-ms conditional prepulses varying from −160 mV to −20 mV with 10-mV increments. The membrane holding potential was −150 mV, and the pulse rate was 0.1 Hz. The dotted line and dashed-dotted line correspondingly represent in the absence and presence of 5 μM EPA for the steady-state inactivation of the wild type of hH1α Na+ channels. The data were fit with a Boltzmann equation.
Figure 7
Voltage-dependent suppression of INa by EPA in HEK293t cells coexpressing N406K and the β1 subunit of hH1α Na+ channels. (A) Whole-cell currents were normalized by INa recorded with the conditioning voltage of −180 mV for their corresponding controls. The experimental protocol is shown in the Inset. Currents were elicited by 10-ms test pulses to 30 mV following a 10-s conditioning pulse varying from −180 mV to −50 mV with 10-mV increments. A 100-ms interval was inserted between the conditioning pulse and the test pulse. The membrane potential was held at −150 mV, and the pulse rate was 0.1 Hz. EPA at 5 μM did not show a significantly voltage-dependent suppression of INa for N406K alone or N406K plus β1 with the protocol of a 100-ms recovery interval. (B) Voltage-dependent suppression of INa in the presence of 5 μM EPA is shown. The Inset is the voltage protocol with a recovery interval of 5 ms. With this protocol, 5 μM EPA did not produce a significant voltage-dependent suppression of INa in HEK293t cells coexpressing N406K and β1. The data were fit with a Boltzmann equation. Each data point represents the average value of at least eight individual cells.
Similar articles
- Coexpression with beta(1)-subunit modifies the kinetics and fatty acid block of hH1(alpha) Na(+) channels.
Xiao YF, Wright SN, Wang GK, Morgan JP, Leaf A. Xiao YF, et al. Am J Physiol Heart Circ Physiol. 2000 Jul;279(1):H35-46. doi: 10.1152/ajpheart.2000.279.1.H35. Am J Physiol Heart Circ Physiol. 2000. PMID: 10899039 - Point mutations in alpha-subunit of human cardiac Na+ channels alter Na+ current kinetics.
Xiao YF, Ke Q, Wang SY, Yang Y, Wang GK, Morgan JP, Leaf A. Xiao YF, et al. Biochem Biophys Res Commun. 2001 Feb 16;281(1):45-52. doi: 10.1006/bbrc.2001.4309. Biochem Biophys Res Commun. 2001. PMID: 11178958 - Potent block of inactivation-deficient Na+ channels by n-3 polyunsaturated fatty acids.
Xiao YF, Ma L, Wang SY, Josephson ME, Wang GK, Morgan JP, Leaf A. Xiao YF, et al. Am J Physiol Cell Physiol. 2006 Feb;290(2):C362-70. doi: 10.1152/ajpcell.00296.2005. Epub 2005 Oct 5. Am J Physiol Cell Physiol. 2006. PMID: 16207794 - Fatty acids suppress voltage-gated Na+ currents in HEK293t cells transfected with the alpha-subunit of the human cardiac Na+ channel.
Xiao YF, Wright SN, Wang GK, Morgan JP, Leaf A. Xiao YF, et al. Proc Natl Acad Sci U S A. 1998 Mar 3;95(5):2680-5. doi: 10.1073/pnas.95.5.2680. Proc Natl Acad Sci U S A. 1998. PMID: 9482947 Free PMC article. - Molecular properties of brain sodium channels: an important target for anticonvulsant drugs.
Catterall WA. Catterall WA. Adv Neurol. 1999;79:441-56. Adv Neurol. 1999. PMID: 10514834 Review.
Cited by
- Incorporated fish oil fatty acids prevent action potential shortening induced by circulating fish oil fatty acids.
Den Ruijter HM, Verkerk AO, Coronel R. Den Ruijter HM, et al. Front Physiol. 2010 Nov 22;1:149. doi: 10.3389/fphys.2010.00149. eCollection 2010. Front Physiol. 2010. PMID: 21423389 Free PMC article. - Eicosapentaenoic acid inhibits voltage-gated sodium channels and invasiveness in prostate cancer cells.
Nakajima T, Kubota N, Tsutsumi T, Oguri A, Imuta H, Jo T, Oonuma H, Soma M, Meguro K, Takano H, Nagase T, Nagata T. Nakajima T, et al. Br J Pharmacol. 2009 Feb;156(3):420-31. doi: 10.1111/j.1476-5381.2008.00059.x. Epub 2009 Jan 16. Br J Pharmacol. 2009. PMID: 19154441 Free PMC article. - Genetic dissection of polyunsaturated fatty acid synthesis in Caenorhabditis elegans.
Watts JL, Browse J. Watts JL, et al. Proc Natl Acad Sci U S A. 2002 Apr 30;99(9):5854-9. doi: 10.1073/pnas.092064799. Epub 2002 Apr 23. Proc Natl Acad Sci U S A. 2002. PMID: 11972048 Free PMC article. - Polyunsaturated Fatty acids modify the gating of kv channels.
Moreno C, Macias A, Prieto A, De La Cruz A, Valenzuela C. Moreno C, et al. Front Pharmacol. 2012 Sep 10;3:163. doi: 10.3389/fphar.2012.00163. eCollection 2012. Front Pharmacol. 2012. PMID: 22973228 Free PMC article. - In-Depth Study of the Interaction, Sensitivity, and Gating Modulation by PUFAs on K+ Channels; Interaction and New Targets.
Moreno C, de la Cruz A, Valenzuela C. Moreno C, et al. Front Physiol. 2016 Nov 24;7:578. doi: 10.3389/fphys.2016.00578. eCollection 2016. Front Physiol. 2016. PMID: 27933000 Free PMC article. Review.
References
- Leaf A, Kang J K, Xiao Y-F, Billman G E, Voskuyl R A. J Membr Biol. 1999;172:1–11. - PubMed
- Xiao Y-F, Wright S N, Wang G K, Morgan J P, Leaf A. Am J Physiol. 2000;279:H35–H46. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials