Signalling via vascular endothelial growth factor receptor-3 is sufficient for lymphangiogenesis in transgenic mice - PubMed (original) (raw)
. 2001 Mar 15;20(6):1223-31.
doi: 10.1093/emboj/20.6.1223.
L Jussila, T Makinen, T Karpanen, M Jeltsch, T V Petrova, H Kubo, G Thurston, D M McDonald, M G Achen, S A Stacker, K Alitalo
Affiliations
- PMID: 11250889
- PMCID: PMC145532
- DOI: 10.1093/emboj/20.6.1223
Signalling via vascular endothelial growth factor receptor-3 is sufficient for lymphangiogenesis in transgenic mice
T Veikkola et al. EMBO J. 2001.
Abstract
Vascular endothelial growth factor receptor-3 (VEGFR-3) has an essential role in the development of embryonic blood vessels; however, after midgestation its expression becomes restricted mainly to the developing lymphatic vessels. The VEGFR-3 ligand VEGF-C stimulates lymphangiogenesis in transgenic mice and in chick chorioallantoic membrane. As VEGF-C also binds VEGFR-2, which is expressed in lymphatic endothelia, it is not clear which receptors are responsible for the lymphangiogenic effects of VEGF-C. VEGF-D, which binds to the same receptors, has been reported to induce angiogenesis, but its lymphangiogenic potential is not known. In order to define the lymphangiogenic signalling pathway we have created transgenic mice overexpressing a VEGFR-3-specific mutant of VEGF-C (VEGF-C156S) or VEGF-D in epidermal keratinocytes under the keratin 14 promoter. Both transgenes induced the growth of lymphatic vessels in the skin, whereas the blood vessel architecture was not affected. Evidence was also obtained that these growth factors act in a paracrine manner in vivo. These results demonstrate that stimulation of the VEGFR-3 signal transduction pathway is sufficient to induce specifically lymphangiogenesis in vivo.
Figures
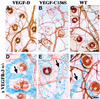
Fig. 1. Receptor specificity and expression of the transgene-encoded proteins. (A) Receptor binding analysis of metabolically labelled VEGF-C, VEGF-D and VEGF-C156S using soluble human or mouse VEGFR-2 and VEGFR-3. (B) Immunoprecipitation and phosphotyrosine analysis of VEGFR-2 and VEGFR-3 after stimulation of primary dermal microvascular endothelial cells with starvation medium, VEGF-D or VEGF-C156S. The asterisk denotes an intracellular VEGFR-3 precursor, which is not phosphorylated upon stimulation. (C) Transgene mRNA expression in K14-VEGF-D and K14-VEGF-C156S mice, and the levels of VEGFR-2 and VEGFR-3 mRNA were determined by northern blotting and hybridization of total skin RNA with the specific probes. WT, wild type; TG, transgenic mouse.
Fig. 2. Lymphatic hyperplasia induced by transgene overexpression. Tissue sections from K14-VEGF-D (A), K14-VEGF-C156S (B), K14-VEGF-C (C) or wild-type (D) skin were immunostained for mouse VEGFR-3. Transgenic skin contains large spaces lined with a VEGFR-3-positive endothelium (A–C), whereas only a few lymphatic capillaries are seen in wild-type skin (D). Scale bar in (D) for (A–D), 50 µm.
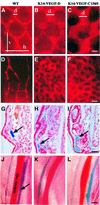
Fig. 3. Immunohistochemical analysis of transgenic skin. High magnification via Nomarski optics. Note that the VEGFR-3-positive endothelial cells associated with the hair follicles (hf) in both wild-type and transgenic skin are also positive for the lymphatic marker LYVE-1 (A, B, F, G, K and L). Also, the endothelium lining the hyperplastic vessels was positive for VEGFR-2 in the K14-VEGF-D mice (C), whereas the hyperplastic vessels of the K14-VEGF-C156S mice and wild-type lymphatic capillaries were negative (H and M). Hair follicles stained for VEGF-C (D, I and N) and VEGF-D (E, J and O). Scale bar in (O) for (A–O), 15 µm.
Fig. 4. Whole-mount analysis of skin blood and lymphatic vasculature. Blood vessels were visualized by injecting the mice intravenously with biotin-labelled L.esculentum lectin followed by vascular perfusion (A–F). Lymphatic vessels were stained blue (arrows) in the skin of K14-VEGF-D and K14-VEGF-C156S mice crossed with heterozygous mutant VEGFR-3+/LacZ mice (D–F). Scale bar in (C) for (A–C), 75 µm; in (F) for (D–F), 65 µm.
Fig. 5. Functional analysis of the lymphatic vessels of normal and transgenic skin. The lymphatic capillaries were visualized by an intradermal injection of TRITC-conjugated dextran into the tail (A–C) or ear (D–F). Ferritin was used as a marker for lymphatic colloidal transport in histological sections from the ear (arrows in G–I). Lymphatic vessels adjacent to the ischiatic vein after injection of Evans Blue into the hind footpads (J–L). Scale bar in (C) for (A–C), 170 µm; in (F) for (D–F), 270 µm; in (I) for (G–I), 80 µm; and in (L) for (J–L), 1250 µm. d, diameter; h, horizontal mesh size; v, vertical mesh size.
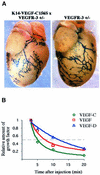
Fig. 6. Lack of a visceral phenotype and a short systemic half-life of transgene encoded proteins. (A) Pericardial lymphatic vessels of a K14-VEGF-C156S × VEGFR-3+/LacZ compound heterozygous and a VEGFR-3+/LacZ mouse were analysed by β-galactoside staining. (B) Relative amounts of VEGF-C, VEGF-D and VEGF in mouse circulation as a function of time after an intravenous injection of recombinant protein. The dashed line denotes 1/2 of the maximum growth factor concentration just after injection.
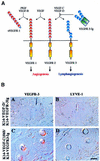
Fig. 7. Lymphatic hyperplasia is neutralized in double transgenic mice expressing soluble VEGFR-3-Ig. (A) Schematic presentation of the binding patterns of the transgene-encoded proteins and endogenous VEGF-VEGFR family members in the skin. (B) Immunohistochemical staining of K14-VEGF-D × VEGFR-3-Ig and K14-VEGF-C156S × VEGFR-3-Ig double transgenic skin for VEGFR-3 and LYVE-1 (A–D). Note the lack of lymphatic vessels. Scale bar in (D) for (A–D), 15 µm.
Similar articles
- VEGF and VEGF-C: specific induction of angiogenesis and lymphangiogenesis in the differentiated avian chorioallantoic membrane.
Oh SJ, Jeltsch MM, Birkenhäger R, McCarthy JE, Weich HA, Christ B, Alitalo K, Wilting J. Oh SJ, et al. Dev Biol. 1997 Aug 1;188(1):96-109. doi: 10.1006/dbio.1997.8639. Dev Biol. 1997. PMID: 9245515 - Vascular endothelial growth factors C and D and their VEGFR-2 and 3 receptors in blood and lymphatic vessels in healthy and arthritic synovium.
Paavonen K, Mandelin J, Partanen T, Jussila L, Li TF, Ristimaki A, Alitalo K, Konttinen YT. Paavonen K, et al. J Rheumatol. 2002 Jan;29(1):39-45. J Rheumatol. 2002. PMID: 11824969 - A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases.
Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, Saksela O, Kalkkinen N, Alitalo K. Joukov V, et al. EMBO J. 1996 Jan 15;15(2):290-98. EMBO J. 1996. PMID: 8617204 Free PMC article. - Lymphatic versus blood vascular endothelial growth factors and receptors in humans.
Partanen TA, Paavonen K. Partanen TA, et al. Microsc Res Tech. 2001 Oct 15;55(2):108-21. doi: 10.1002/jemt.1162. Microsc Res Tech. 2001. PMID: 11596156 Review. - [Lymphangiogenesis and biological activity ov vascular endothelial growth factor-C].
Mandriota SJ, Pepper MS. Mandriota SJ, et al. J Soc Biol. 1999;193(2):159-63. J Soc Biol. 1999. PMID: 10451350 Review. French.
Cited by
- Help-me signaling: Non-cell autonomous mechanisms of neuroprotection and neurorecovery.
Xing C, Lo EH. Xing C, et al. Prog Neurobiol. 2017 May;152:181-199. doi: 10.1016/j.pneurobio.2016.04.004. Epub 2016 Apr 11. Prog Neurobiol. 2017. PMID: 27079786 Free PMC article. Review. - Transgenic overexpression of VEGF-C induces weight gain and insulin resistance in mice.
Karaman S, Hollmén M, Yoon SY, Alkan HF, Alitalo K, Wolfrum C, Detmar M. Karaman S, et al. Sci Rep. 2016 Aug 11;6:31566. doi: 10.1038/srep31566. Sci Rep. 2016. PMID: 27511834 Free PMC article. - Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3.
Mäkinen T, Veikkola T, Mustjoki S, Karpanen T, Catimel B, Nice EC, Wise L, Mercer A, Kowalski H, Kerjaschki D, Stacker SA, Achen MG, Alitalo K. Mäkinen T, et al. EMBO J. 2001 Sep 3;20(17):4762-73. doi: 10.1093/emboj/20.17.4762. EMBO J. 2001. PMID: 11532940 Free PMC article. - Hemostasis stimulates lymphangiogenesis through release and activation of VEGFC.
Lim L, Bui H, Farrelly O, Yang J, Li L, Enis D, Ma W, Chen M, Oliver G, Welsh JD, Kahn ML. Lim L, et al. Blood. 2019 Nov 14;134(20):1764-1775. doi: 10.1182/blood.2019001736. Blood. 2019. PMID: 31562136 Free PMC article. - Distinct roles of vascular endothelial growth factor-D in lymphangiogenesis and metastasis.
Kopfstein L, Veikkola T, Djonov VG, Baeriswyl V, Schomber T, Strittmatter K, Stacker SA, Achen MG, Alitalo K, Christofori G. Kopfstein L, et al. Am J Pathol. 2007 Apr;170(4):1348-61. doi: 10.2353/ajpath.2007.060835. Am J Pathol. 2007. PMID: 17392173 Free PMC article.
References
- Achen M.G. et al. (2000) Monoclonal antibodies to vascular endothelial growth factor-D block its interactions with both VEGF receptor-2 and VEGF receptor-3. Eur. J. Biochem., 267, 2505–2515. - PubMed
- Aiello L.P., Pierce,E.A., Foley,E.D., Takagi,H., Chen,H., Riddle,L., Ferrara,N., King,G.L. and Smith,L.E. (1995) Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc. Natl Acad. Sci. USA, 92, 10457–10461. - PMC - PubMed
- Byrne C., Tainsky,M. and Fuchs,E. (1994) Programming gene expression in developing epidermis. Development, 120, 2369–2383. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous