Kinetic analysis of translocation through nuclear pore complexes - PubMed (original) (raw)
Kinetic analysis of translocation through nuclear pore complexes
K Ribbeck et al. EMBO J. 2001.
Abstract
The mechanism of facilitated translocation through nuclear pore complexes (NPCs) is only poorly understood. Here, we present a kinetic analysis of the process using various model substrates. We find that the translocation capacity of NPCs is unexpectedly high, with a single NPC allowing a mass flow of nearly 100 MDa/s and rates in the order of 10(3) translocation events per second. Our data further indicate that high affinity interactions between the translocation substrate and NPC components are dispensable for translocation. We propose a 'selective phase model' that could explain how NPCs function as a permeability barrier for inert molecules and yet become selectively permeable for nuclear transport receptors and receptor-cargo complexes.
Figures
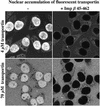
Fig. 1. Transportin—a simple model substrate for facilitated translocation through NPCs. Alexa-labelled transportin (4 or 70 µM) was added to permeabilized cells and its distribution subsequently recorded by scanning with a confocal microscope through the unfixed samples. One minute was sufficient for a clear nuclear accumulation of transportin (left). The dominant-negative importin β 45–462 mutant (3 µM) blocked the transportin influx completely (right).
Fig. 2. Time course of transportin influx into nuclei. Fluorescent transportin (4 µM) was added to the permeabilized cells and its influx into nuclei recorded in real time by confocal microscopy. (A) Frames taken at indicated time points. (B) Integrated nuclear fluorescence (excluding the NPC signal) is plotted against time. The time course fits an exponential curve of the form _f_[_t_] = _f_Max(1 – e–kt), where t is time, _f_[_t_] is the nuclear fluorescence, _f_Max is the endpoint of the reaction and k is the first-order rate constant. For conversion of fluorescence intensities into absolute substrate concentration see Materials and methods. The initial rate of nuclear accumulation (given by _r_i = _kf_Max) for 4 µM transportin was found to be 0.6 µM/s, corresponding to ∼150 translocations·NPC–1·s–1 (average from 10 independent measurements).
Fig. 2. Time course of transportin influx into nuclei. Fluorescent transportin (4 µM) was added to the permeabilized cells and its influx into nuclei recorded in real time by confocal microscopy. (A) Frames taken at indicated time points. (B) Integrated nuclear fluorescence (excluding the NPC signal) is plotted against time. The time course fits an exponential curve of the form _f_[_t_] = _f_Max(1 – e–kt), where t is time, _f_[_t_] is the nuclear fluorescence, _f_Max is the endpoint of the reaction and k is the first-order rate constant. For conversion of fluorescence intensities into absolute substrate concentration see Materials and methods. The initial rate of nuclear accumulation (given by _r_i = _kf_Max) for 4 µM transportin was found to be 0.6 µM/s, corresponding to ∼150 translocations·NPC–1·s–1 (average from 10 independent measurements).
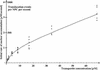
Fig. 3. Concentration dependence of transportin flux through NPCs. The initial rates of influx of fluorescent transportin were determined for concentrations of 0.85–68 µM (42 data points). A saturation of influx by increasing transportin concentrations is clearly evident. However, the dose dependence deviates from an ideal Michaelis–Menten curve of the form v = V_Max_c/(_K_M + c), apparently because cooperativity in NPC passage becomes significant at higher transportin concentration. The fit was improved by introducing a correction factor (1 + k_2_c), which takes cooperativity into account and gives a modified Michaelis–Menten equation of the form
The numerically obtained best-fit parameters are: _V_Max = 300 events·NPC–1·s–1; _K_M = 4 µM; _k_2 = 0.03 µM–1.
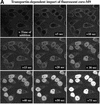
Fig. 4. Rate of core–M9 import into nuclei. The pentameric core–M9 fusion (Alexa labelled) was complexed with transportin at a 1:5 molar ratio. Import into nuclei was with 1 µM of this 630 kDa complex in the presence of Ran and an energy-regenerating system. (A) Time lapse images of M9 import. (B) Plot of integrated nuclear fluorescence (nuclear core–M9 concentration) versus time. The initial rate of nuclear accumulation was 0.11 µM/s, corresponding to 28 translocations·NPC–1·s–1 or a mass flow of ∼17 MDa·NPC–1·s–1.
Fig. 4. Rate of core–M9 import into nuclei. The pentameric core–M9 fusion (Alexa labelled) was complexed with transportin at a 1:5 molar ratio. Import into nuclei was with 1 µM of this 630 kDa complex in the presence of Ran and an energy-regenerating system. (A) Time lapse images of M9 import. (B) Plot of integrated nuclear fluorescence (nuclear core–M9 concentration) versus time. The initial rate of nuclear accumulation was 0.11 µM/s, corresponding to 28 translocations·NPC–1·s–1 or a mass flow of ∼17 MDa·NPC–1·s–1.
Fig. 5. Comparison of NTF2 flux through NPCs with that of GFP. (A) Comparison of influx of GFP (5 µM), wild-type NTF2 (2.5 µM dimers) and the NTF2 W7R mutant into nuclei. Note that NTF2 nearly instantaneously equilibrated between nucleus and cytoplasm, while GFP remained excluded from the nuclei when tested on the same time scale. Endpoints were 2 min for GFP, 12 s for wild-type NTF2 and 60 s for the NTF2 W7R mutant. (B) Quantitation of NTF2 (wild type) influx into nuclei. Equilibration between the nuclear and cytoplasmic NTF2 pools occurs with a first-order rate constant of k = 1 s–1 or a half-time of ∼0.7 s. This implies that a nucleocytoplasmic concentration difference of 2.5 µM causes a change in nuclear NTF2 concentration at an initial rate of 2.5 µM/s, which corresponds to a flux of ∼620 NTF2 dimers·NPC–1·s–1 (for details see Supplementary data).
Fig. 5. Comparison of NTF2 flux through NPCs with that of GFP. (A) Comparison of influx of GFP (5 µM), wild-type NTF2 (2.5 µM dimers) and the NTF2 W7R mutant into nuclei. Note that NTF2 nearly instantaneously equilibrated between nucleus and cytoplasm, while GFP remained excluded from the nuclei when tested on the same time scale. Endpoints were 2 min for GFP, 12 s for wild-type NTF2 and 60 s for the NTF2 W7R mutant. (B) Quantitation of NTF2 (wild type) influx into nuclei. Equilibration between the nuclear and cytoplasmic NTF2 pools occurs with a first-order rate constant of k = 1 s–1 or a half-time of ∼0.7 s. This implies that a nucleocytoplasmic concentration difference of 2.5 µM causes a change in nuclear NTF2 concentration at an initial rate of 2.5 µM/s, which corresponds to a flux of ∼620 NTF2 dimers·NPC–1·s–1 (for details see Supplementary data).
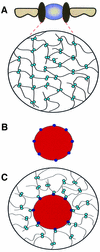
Fig. 6. The selective phase model for facilitated translocation through NPCs. (A) The permeability barrier of the central plug ‘at rest’. Phe-rich nucleoporin repeats (light blue circles) attract each other and form a meshwork that restricts passage of inert objects. (B) An object capable of facilitated translocation, distinguished from inert objects by binding sites (dark blue circles) for the Phe-rich repeats. (C) The translocating species interacts with the Phe-rich repeats and thus becomes part of the meshwork. It dissolves in the central plug, which allows crossing of the permeability barrier.
Similar articles
- Nuclear transport of single molecules: dwell times at the nuclear pore complex.
Kubitscheck U, Grünwald D, Hoekstra A, Rohleder D, Kues T, Siebrasse JP, Peters R. Kubitscheck U, et al. J Cell Biol. 2005 Jan 17;168(2):233-43. doi: 10.1083/jcb.200411005. J Cell Biol. 2005. PMID: 15657394 Free PMC article. - Nuclear protein import is reduced in cells expressing nuclear envelopathy-causing lamin A mutants.
Busch A, Kiel T, Heupel WM, Wehnert M, Hübner S. Busch A, et al. Exp Cell Res. 2009 Aug 15;315(14):2373-85. doi: 10.1016/j.yexcr.2009.05.003. Epub 2009 May 12. Exp Cell Res. 2009. PMID: 19442658 - Characterization of Ran-driven cargo transport and the RanGTPase system by kinetic measurements and computer simulation.
Görlich D, Seewald MJ, Ribbeck K. Görlich D, et al. EMBO J. 2003 Mar 3;22(5):1088-100. doi: 10.1093/emboj/cdg113. EMBO J. 2003. PMID: 12606574 Free PMC article. - Dynamic nuclear pore complexes: life on the edge.
Tran EJ, Wente SR. Tran EJ, et al. Cell. 2006 Jun 16;125(6):1041-53. doi: 10.1016/j.cell.2006.05.027. Cell. 2006. PMID: 16777596 Review. - Should I stay or should I go? Nucleocytoplasmic trafficking in plant innate immunity.
Wiermer M, Palma K, Zhang Y, Li X. Wiermer M, et al. Cell Microbiol. 2007 Aug;9(8):1880-90. doi: 10.1111/j.1462-5822.2007.00962.x. Epub 2007 May 15. Cell Microbiol. 2007. PMID: 17506817 Review.
Cited by
- Effect of charge, hydrophobicity, and sequence of nucleoporins on the translocation of model particles through the nuclear pore complex.
Tagliazucchi M, Peleg O, Kröger M, Rabin Y, Szleifer I. Tagliazucchi M, et al. Proc Natl Acad Sci U S A. 2013 Feb 26;110(9):3363-8. doi: 10.1073/pnas.1212909110. Epub 2013 Feb 12. Proc Natl Acad Sci U S A. 2013. PMID: 23404701 Free PMC article. - Nucleocytoplasmic transport: a role for nonspecific competition in karyopherin-nucleoporin interactions.
Tetenbaum-Novatt J, Hough LE, Mironska R, McKenney AS, Rout MP. Tetenbaum-Novatt J, et al. Mol Cell Proteomics. 2012 May;11(5):31-46. doi: 10.1074/mcp.M111.013656. Epub 2012 Feb 22. Mol Cell Proteomics. 2012. PMID: 22357553 Free PMC article. - In vivo analysis of human nucleoporin repeat domain interactions.
Xu S, Powers MA. Xu S, et al. Mol Biol Cell. 2013 Apr;24(8):1222-31. doi: 10.1091/mbc.E12-08-0585. Epub 2013 Feb 20. Mol Biol Cell. 2013. PMID: 23427268 Free PMC article. - The structure of the NXF2/NXT1 heterodimeric complex reveals the combined specificity and versatility of the NTF2-like fold.
Kerkow DE, Carmel AB, Menichelli E, Ambrus G, Hills RD Jr, Gerace L, Williamson JR. Kerkow DE, et al. J Mol Biol. 2012 Jan 27;415(4):649-65. doi: 10.1016/j.jmb.2011.11.027. Epub 2011 Nov 22. J Mol Biol. 2012. PMID: 22123199 Free PMC article. - The dynamic pathway of nuclear RNA in eukaryotes.
Sheinberger J, Shav-Tal Y. Sheinberger J, et al. Nucleus. 2013 May-Jun;4(3):195-205. doi: 10.4161/nucl.24434. Epub 2013 Apr 11. Nucleus. 2013. PMID: 23580182 Free PMC article. Review.
References
- Bayliss R., Ribbeck,K., Akin,D., Kent,H.M., Feldherr,C.M., Görlich,D. and Stewart,M. (1999) Interaction between NTF2 and xFxFG-containing nucleoporins is required to mediate nuclear import of RanGDP. J. Mol. Biol., 293, 579–593. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources