Dynamic movements of organelles containing Niemann-Pick C1 protein: NPC1 involvement in late endocytic events - PubMed (original) (raw)
Dynamic movements of organelles containing Niemann-Pick C1 protein: NPC1 involvement in late endocytic events
D C Ko et al. Mol Biol Cell. 2001 Mar.
Free PMC article
Abstract
People homozygous for mutations in the Niemann-Pick type C1 (NPC1) gene have physiological defects, including excess accumulation of intracellular cholesterol and other lipids, that lead to drastic neural and liver degeneration. The NPC1 multipass transmembrane protein is resident in late endosomes and lysosomes, but its functions are unknown. We find that organelles containing functional NPC1-fluorescent protein fusions undergo dramatic movements, some in association with extending strands of endoplasmic reticulum. In NPC1 mutant cells the NPC1-bearing organelles that normally move at high speed between perinuclear regions and the periphery of the cell are largely absent. Pulse-chase experiments with dialkylindocarbocyanine low-density lipoprotein showed that NPC1 organelles function late in the endocytic pathway; NPC1 protein may aid the partitioning of endocytic and lysosomal compartments. The close connection between NPC1 and the drug U18666A, which causes NPC1-like organelle defects, was established by rescuing drug-treated cells with overproduced NPC1. U18666A inhibits outward movements of NPC1 organelles, trapping membranes and cholesterol in perinuclear organelles similar to those in NPC1 mutant cells, even when cells are grown in lipoprotein-depleted serum. We conclude that NPC1 protein promotes the creation and/or movement of particular late endosomes, which rapidly transport materials to and from the cell periphery.
Figures
Figure 1
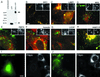
NPC1-FP is correctly localized in CT60 CHO cells and is functional in clearing accumulated cholesterol. Extracts of CT60 cells expressing GFP or NPC1-YFP were resolved on a 4–12% gradient gel and probed with an anti-GFP antibody (A). The NPC1-YFP stable line produces predominant bands near 200 kDa (arrow), consistent with the glycosylated forms of NPC1-YFP (predicted molecular weight for nonglycosylated fusion = 170 kDa). A faint smaller band (arrowhead), presumably a cleavage product, is also present, but the colocalization of NPC1-GFP with anti-NPC1 antibody staining (B) indicates that the tag stays largely attached to NPC1. Bar, 5 μm. Cells expressing NPC1-YFP were antibody stained with various primary antibodies, followed by incubation with CY5-conjugated secondary antibody, and imaged using the appropriate filter sets. The tagged protein localizes to organelles that contain LAMP1 (C) but do not contain the cation-independent MPR (D). To determine whether the protein is localizing only to this compartment, other markers were examined, including PMP70 (E), caveolin (F), βCOP (G), and VAMP7-FP (H). CT60 cells were transiently transfected with NPC1 and GFP (I) or NPC1-GFP (J) and the amount of free cholesterol was evaluated by filipin staining in each case. NPC1-GFP is able to clear the accumulated cholesterol as well as untagged NPC1 (97 ± 1 versus 95 ± 2% of expressing cells rescued). GFP alone has no affect on the accumulated cholesterol (3.5 ± 0.5%). Bar, 5 μm.
Figure 2
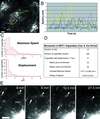
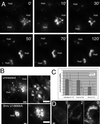
NPC1-FP localizes to moving structures that are variable in their rates and direction. NPC1-FP was observed in punctate and tubulovesicular structures that moved both toward and away from the perinuclear region at a broad range of speeds. The paths of several structures over 30 s are shown in A, with the number of the path next to the organelle's initial position. The instantaneous speeds of the tracked particles are plotted versus time, demonstrating the stop-and-go nature of the movements (B). We examined the movements of 200 randomly chosen structures from 10 cells and characterized the movements based on maximum speed, displacement, and direction (C and D). Large NPC1-FP rings move toward the nucleus while simultaneously decreasing in size (E). Images taken every 30 s for 30 min revealed that structures that appeared stably localized over shorter intervals, undergo a retraction toward the perinuclear region. Two retracting rings are pointed out with an arrow and arrowhead. Note the changing tubular structures extending from the rings, particularly at 6 min (asterisks). Bar, 5 μ m. Video 1 accompanies Figure 2A. NPC1-FP localizes to moving structures that are variable in their rates and direction. Images were acquired every second for 30 s and played back at a rate of six images per second. Video 2 accompanies Figure 2E. Slow retraction of large NPC1-FP rings. Images were acquired every 30 s for 30 min and played back at a rate of six images per second.
Figure 3
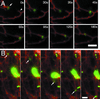
NPC1-FP vesicles associate with and move with the ER. CT60 cells stably transfected with NPC1-YFP were transiently transfected with Sec61beta-CFP, an ER-resident protein. Twenty-four hours posttransfection, the two proteins were visualized by sequential exposures (0.5 s CFP and 1 s YFP) taken every 5 s. Images at each interval were merged and the relative motions of the labeled structures were examined. (A) The eight frames show an organelle with NPC1-YFP that is localized at the end of an ER segment (arrow). The two move a distance of ∼3 μm to associate with another ER segment (arrowhead) (0–55 s). The vesicle and ER then appear to retract along this second segment of ER until resting at the junction between the two segments (55–180 s). (B) An NPC1-FP organelle (arrow) moves a distance of ∼16 μm while pulling a segment of ER with it. Bar, 2 μm. Video 3 accompanies Figure 3A. NPC1-YFP organelles associate with the ends of ER segments labeled with Sec61β-CFP. Sequential CFP and YFP images were acquired every 5 s for 3 min and played back at a rate of six images per second. Video 4 accompanies Figure 3B. NPC1-YFP organelles appear to pull middle segments of ER labeled with Sec61β-CFP. Sequential CFP and YFP images were acquired every 5 s for1 min and played back at a rate of six images per second.
Figure 4
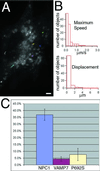
NPC1-containing organelles display reduced movement in cells lacking functional NPC1. To monitor the movements of the NPC1-containing compartment in cells lacking functional NPC1, VAMP7-FP or NPC1(P692S)-FP was transiently transfected into CT60 cells to mark the abnormal organelles. Representative tracks for VAMP7-FP are shown in A. Quantification of the movement of 100 randomly chosen organelles from five cells (B) shows that the speed and displacement of these organelles are severely decreased in CT60 cells. Observations of all the fluorescent organelles within cells showed that the fraction engaged in vectorial transport is decreased in cells lacking functional NPC1 or expressing an NPC1 mutant with reduced function (P692S) (C). Bar, 5 μ m. Video 5 accompanies Figure 4A. Reduced movement of VAMP7-FP organelles in NPC1 mutant cells. Images were acquired every second for 1 min and played back at a rate of six images per second.
Figure 5
NPC1-FP organelles participate in late events in the endocytic pathway. Pulse-chase experiments at 37°C with DiI LDL revealed the kinetics of entry into the NPC1-containing compartment. CT60 cells transiently transfected with NPC1-GFP or VAMP7-GFP were labeled with DiI-LDL (30 μM) for 3 min and then fixed following a 3-, 10-, 30-, and 120-min chase period. The fraction of all DiI-labeled structures that colocalized with NPC1-GFP, VAMP7-GFP, or only the enlarged VAMP7-GFP organelles was quantified for 10 cells at each time point (A). DiI shows significant colocalization with NPC1-GFP or VAMP7-GFP at 10 min and later time points, indicative of NPC1-FP being present in late endosomes and the terminal endocytic compartment. The enlarged VAMP-GFP organelles colocalize with DiI beginning at 30 min, but the bulk of the DiI has not reached these organelles until 2 h (C). In living cells, the fraction of all DiI-labeled structures engaged in vectorial movement (displacement >1.5 μm) was quantified at 10, 30, and 120 min (B). Similar amounts of movement were seen early in the endocytic pathway (10 and 30 min) regardless of the presence of NPC1. Following these normal early movements, loss of NPC1 prevents further DiI movement (2 h) and results in the sequestration of DiI in the cholesterol-laden organelles. Bar, 5 μm.
Figure 6
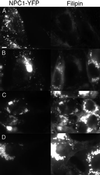
Overexpression of NPC1 results in decreased sensitivity to U18666A. NPC1-YFP expressing cells were plated with 25RA cells and treated for 24 h with 0, 0.3, 3, and 10 μM U18666A (A–D). YFP fluorescence is shown on the left and filipin staining on the right. The low concentration of U18666A (0.3 μM) is able to induce cholesterol accumulation in 25RA cells but not in the cells overexpressing NPC1-YFP. Increasing the U18666A is able to overcome this suppression, as shown with intermediate (3 μM) and high concentrations (10 μM) of the drug. Bar, 5 μm.
Figure 7
The formation of cholesterol-laden organelles in cells treated with U18666A. Cells were incubated with 10 μM U18666A and imaged every 30 s for the first 2 h of treatment (A). By 2 h, all cells displayed a prominent perinuclear aggregate formed from the loosely associated, dynamic perinuclear cluster of NPC1 organelles present in untreated cells. (B) Images (100) taken over 25 s have been merged to show the tracks of movement into and out of this perinuclear cluster in untreated cells and cells treated for 3 h with U18666A. Quantification of the movement to and from the perinuclear aggregate (C) reveals decreased movement out of the aggregate at 1–1.5 h and decreased movement in both directions by 2.5–3.5 h. The effects of U18666A did not require the presence of exogenous sources of cholesterol as shown by filipin staining in D. Cells incubated in F12 without serum for 48 h (D1) still formed enlarged cholesterol-laden organelles when incubated for the final 24 h with U18666A (D2), though the overall amount of cholesterol was not as high as that seen in cells grown in F12 (+10% fetal bovine serum) for 24 h with U18666A (D3). nuc, nucleus; bar, 5 μm. Video 7 accompanies Figure 7B untreated. Movements of perinuclear NPC1-FP organelles in untreated cells. Images were acquired approximately every 0.25 s for 50 s and played back at a rate of 15 images per second. Video 8 accompanies Figure 7B 3 h U18666A. Decreased movements of perinuclear NPC1-FP organelles in cells following U18666A treatment. Images were acquired approximately every 0.25 s for 25 s and played back at a rate of 15 images per second. Video 6 accompanies Figure 7A. The formation of abnormal organelles in cells treated with U18666A. Images were acquired every 30 s for 2 h and played back at a rate of six images per second.
Figure 8
Some vesicular traffic continues out of cholesterol-laden organelles in NPC1 mutant cells. Images from CT60 cells transiently transfected with VAMP7-YFP were taken using streaming acquisition. VAMP7 localizes to the large abnormal organelles found in NPC, as well as VAMP7-positive, NPC1-negative vesicles that continue to move rapidly without functional NPC1. The arrow at 0.6 s points at a bright VAMP7 spot. At 4.6 s, this structure begins to extend off of the parent organelle. Between 5.5 and 5.7 s, what appears to be a fission event occurs as the tubule is severed. Bar, 1 μm. Video 9 accompanies Figure 8. Continued movement of VAMP7-positive/NPC1-negative vesicles out of cholesterol-laden organelles. Images were acquired every 0.25 s for 6 s and were played back at a rate of four images per second.
Similar articles
- Cessation of rapid late endosomal tubulovesicular trafficking in Niemann-Pick type C1 disease.
Zhang M, Dwyer NK, Love DC, Cooney A, Comly M, Neufeld E, Pentchev PG, Blanchette-Mackie EJ, Hanover JA. Zhang M, et al. Proc Natl Acad Sci U S A. 2001 Apr 10;98(8):4466-71. doi: 10.1073/pnas.081070898. Proc Natl Acad Sci U S A. 2001. PMID: 11296289 Free PMC article. - Niemann-Pick C1 functions independently of Niemann-Pick C2 in the initial stage of retrograde transport of membrane-impermeable lysosomal cargo.
Goldman SD, Krise JP. Goldman SD, et al. J Biol Chem. 2010 Feb 12;285(7):4983-94. doi: 10.1074/jbc.M109.037622. Epub 2009 Dec 10. J Biol Chem. 2010. PMID: 20007703 Free PMC article. - Depletion of rafts in late endocytic membranes is controlled by NPC1-dependent recycling of cholesterol to the plasma membrane.
Lusa S, Blom TS, Eskelinen EL, Kuismanen E, Månsson JE, Simons K, Ikonen E. Lusa S, et al. J Cell Sci. 2001 May;114(Pt 10):1893-900. doi: 10.1242/jcs.114.10.1893. J Cell Sci. 2001. PMID: 11329376 - Niemann-Pick type C mutations cause lipid traffic jam.
Liscum L. Liscum L. Traffic. 2000 Mar;1(3):218-25. doi: 10.1034/j.1600-0854.2000.010304.x. Traffic. 2000. PMID: 11208105 Review. - Mechanisms and consequences of impaired lipid trafficking in Niemann-Pick type C1-deficient mammalian cells.
Karten B, Peake KB, Vance JE. Karten B, et al. Biochim Biophys Acta. 2009 Jul;1791(7):659-70. doi: 10.1016/j.bbalip.2009.01.025. Epub 2009 Feb 11. Biochim Biophys Acta. 2009. PMID: 19416638 Review.
Cited by
- Intracellular cholesterol transport inhibition Impairs autophagy flux by decreasing autophagosome-lysosome fusion.
Maharjan Y, Dutta RK, Son J, Wei X, Park C, Kwon HM, Park R. Maharjan Y, et al. Cell Commun Signal. 2022 Nov 25;20(1):189. doi: 10.1186/s12964-022-00942-z. Cell Commun Signal. 2022. PMID: 36434621 Free PMC article. - Sequestration of AS-DACA into acidic compartments of the membrane trafficking system as a mechanism of drug resistance in rhabdomyosarcoma.
Williams M, Catchpoole D. Williams M, et al. Int J Mol Sci. 2013 Jun 25;14(7):13042-62. doi: 10.3390/ijms140713042. Int J Mol Sci. 2013. PMID: 23799359 Free PMC article. - Identification of potential immune-related hub genes in Parkinson's disease based on machine learning and development and validation of a diagnostic classification model.
Xin G, Niu J, Tian Q, Fu Y, Chen L, Yi T, Tian K, Sun X, Wang N, Wang J, Zhang H, Wang L. Xin G, et al. PLoS One. 2023 Dec 5;18(12):e0294984. doi: 10.1371/journal.pone.0294984. eCollection 2023. PLoS One. 2023. PMID: 38051734 Free PMC article. - Endosomal accumulation of Toll-like receptor 4 causes constitutive secretion of cytokines and activation of signal transducers and activators of transcription in Niemann-Pick disease type C (NPC) fibroblasts: a potential basis for glial cell activation in the NPC brain.
Suzuki M, Sugimoto Y, Ohsaki Y, Ueno M, Kato S, Kitamura Y, Hosokawa H, Davies JP, Ioannou YA, Vanier MT, Ohno K, Ninomiya H. Suzuki M, et al. J Neurosci. 2007 Feb 21;27(8):1879-91. doi: 10.1523/JNEUROSCI.5282-06.2007. J Neurosci. 2007. PMID: 17314284 Free PMC article. - Genetic perspective on the synergistic connection between vesicular transport, lysosomal and mitochondrial pathways associated with Parkinson's disease pathogenesis.
Smolders S, Van Broeckhoven C. Smolders S, et al. Acta Neuropathol Commun. 2020 May 6;8(1):63. doi: 10.1186/s40478-020-00935-4. Acta Neuropathol Commun. 2020. PMID: 32375870 Free PMC article. Review.
References
- Advani RJ, Bae HR, Bock JB, Chao DS, Doung YC, Prekeris R, Yoo JS, Scheller RH. Seven novel mammalian SNARE proteins localize to distinct membrane compartments. J Biol Chem. 1998;273:10317–10324. - PubMed
- Allan V, Vale R. Movement of membrane tubules along microtubules in vitro: evidence for specialized sites of motor attachment. J Cell Sci. 1994;107:1885–1897. - PubMed
- Blanchette-Mackie EJ, Dwyer NK, Amende LM, Kruth HS, Butler JD, Sokol J, Comly ME, Vanier MT, August JT, Brady RO, et al. Type-C Niemann-Pick disease: low density lipoprotein uptake is associated with premature cholesterol accumulation in the Golgi complex and excessive cholesterol storage in lysosomes. Proc Natl Acad Sci USA. 1988;85:8022–8026. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials