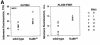Mammalian Ku86 protein prevents telomeric fusions independently of the length of TTAGGG repeats and the G-strand overhang - PubMed (original) (raw)
Mammalian Ku86 protein prevents telomeric fusions independently of the length of TTAGGG repeats and the G-strand overhang
E Samper et al. EMBO Rep. 2000 Sep.
Abstract
Ku86 together with Ku70, DNA-PKcs, XRCC4 and DNA ligase IV forms a complex involved in repairing DNA double-strand breaks (DSB) in mammals. Yeast Ku has an essential role at the telomere; in particular, Ku deficiency leads to telomere shortening, loss of telomere clustering, loss of telomeric silencing and deregulation of the telomeric G-overhang. In mammals, Ku proteins associate to telomeric repeats; however, the possible role of Ku in regulating telomere length has not yet been addressed. We have measured telomere length in different cell types from wild-type and Ku86-deficient mice. In contrast to yeast, Ku86 deficiency does not result in telomere shortening or deregulation of the G-strand overhang. Interestingly, Ku86-/- cells show telomeric fusions with long telomeres (>81 kb) at the fusion point. These results indicate that mammalian Ku86 plays a fundamental role at the telomere by preventing telomeric fusions independently of the length of TTAGGG repeats and the integrity of the G-strand overhang.
Figures
Fig. 1. Chromosome aberrations in Ku86–/– primary MEFs. Cytogenetic alterations detected in Ku86–/– metaphases from primary MEFs after hybridization with DAPI and a fluorescent Cy-3 labelled PNA-telomeric probe. For quantifications see Table I. Blue colour corresponds to chromosome DNA stained with DAPI; yellow and white dots correspond to TTAGGG repeats; red arrows highlight each of the different chromosomal abnormalities shown in the figure.
Fig. 2. Telomere analysis of littermate wild-type and Ku86–/– MEFs by Q-FISH and FLOW-FISH. (A) Telomere fluorescence of littermate wild-type and Ku86–/– MEFs (passage 1) by Q-FISH and FLOW-FISH (Madrid section in Methods). (B) Telomere length distribution of at least 14 000 telomeres in six different littermate wild-type and Ku86–/– primary MEFs. The histogram depicts similar telomeres in Ku86–/– and wild-type cells. 1 TFU corresponds to 1 kb of TTAGGG repeats.
Fig. 2. Telomere analysis of littermate wild-type and Ku86–/– MEFs by Q-FISH and FLOW-FISH. (A) Telomere fluorescence of littermate wild-type and Ku86–/– MEFs (passage 1) by Q-FISH and FLOW-FISH (Madrid section in Methods). (B) Telomere length distribution of at least 14 000 telomeres in six different littermate wild-type and Ku86–/– primary MEFs. The histogram depicts similar telomeres in Ku86–/– and wild-type cells. 1 TFU corresponds to 1 kb of TTAGGG repeats.
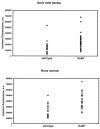
Fig. 3. Telomere length analysis of littermate wild-type and Ku86–/– BM and male premeiotic germ cells by Q-FISH. Telomere fluorescence of littermate wild-type and Ku86–/– nuclei by Q-FISH (Leiden section in Methods). Telomere fluorescence of interphase nuclei from two wild-type and Ku86–/– littermates is represented in arbitrary units; each point represents an individual nucleus. Both bone marrow and diploid premeiotic male germ cell nuclei were analysed. BM: wild-type, 28 nuclei; Ku86–/–, 35 nuclei. Testis: wild-type, 27 nuclei; Ku86–/– 39 nuclei.
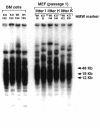
Fig. 4. TRF analysis in wild-type and Ku86–/– primary MEFs and BM cells. Three different wild-type (Wt) and Ku86–/– (KO) littermate MEFs (passage 1) were studied, as well as fresh BM samples from two littermate wild-type (Wt) and Ku86–/– (KO) mice. TRF signals were similar in Ku86–/– and wild-type cells. In the case of MEF cultures: I, H and K are three different litters born from heterozygous parents (see also Tables I and II, and Figure 2 for other analyses of the same MEFs). In the case of BM cells, 162, 163, 164 and 165 are mice from the same litter born from heterozygous parents.
Fig. 5. Normal G-strand overhang in Ku86–/– deficient primary cells. G-strand overhangs in fresh BM cells from two littermate wild-type and Ku86–/– mice are visualized in native gel after hybridization with a (CCCTAA)4 probe (see METHODS). Upon treatment with two different doses of mung bean nuclease (MBN), the G-strand specific signal decreases. (1) and (2) are two different litters. As control, the same gel was denatured and reprobed with the (CCCTAA)4 probe to visualize telomeres.
Fig. 6. Telomerase activity in wild-type and Ku86–/– MEFs. S-100 extracts were prepared from wild-type (A3 and B1) and Ku86–/– (A1 and C7) MEFs and assayed for telomerase activity. Extracts were pretreated (+) or not (–) with RNase. The protein concentration used is indicated. The arrow indicates the internal control (IC) for PCR efficiency.
Similar articles
- Mammalian Ku86 mediates chromosomal fusions and apoptosis caused by critically short telomeres.
Espejel S, Franco S, Rodríguez-Perales S, Bouffler SD, Cigudosa JC, Blasco MA. Espejel S, et al. EMBO J. 2002 May 1;21(9):2207-19. doi: 10.1093/emboj/21.9.2207. EMBO J. 2002. PMID: 11980718 Free PMC article. - Effects of double-strand break repair proteins on vertebrate telomere structure.
Wei C, Skopp R, Takata M, Takeda S, Price CM. Wei C, et al. Nucleic Acids Res. 2002 Jul 1;30(13):2862-70. doi: 10.1093/nar/gkf396. Nucleic Acids Res. 2002. PMID: 12087170 Free PMC article. - Maintenance of double-stranded telomeric repeats as the critical determinant for cell viability in yeast cells lacking Ku.
Gravel S, Wellinger RJ. Gravel S, et al. Mol Cell Biol. 2002 Apr;22(7):2182-93. doi: 10.1128/MCB.22.7.2182-2193.2002. Mol Cell Biol. 2002. PMID: 11884605 Free PMC article. - Telomeres--unsticky ends.
Shore D. Shore D. Science. 1998 Sep 18;281(5384):1818-9. doi: 10.1126/science.281.5384.1818. Science. 1998. PMID: 9776685 Review. No abstract available. - Ku, a DNA repair protein with multiple cellular functions?
Featherstone C, Jackson SP. Featherstone C, et al. Mutat Res. 1999 May 14;434(1):3-15. doi: 10.1016/s0921-8777(99)00006-3. Mutat Res. 1999. PMID: 10377944 Review.
Cited by
- DNA-PK controls Apollo's access to leading-end telomeres.
Sonmez C, Toia B, Eickhoff P, Matei AM, El Beyrouthy M, Wallner B, Douglas ME, de Lange T, Lottersberger F. Sonmez C, et al. Nucleic Acids Res. 2024 May 8;52(8):4313-4327. doi: 10.1093/nar/gkae105. Nucleic Acids Res. 2024. PMID: 38407308 Free PMC article. - A Nested PCR Telomere Fusion Assay Highlights the Widespread End-Capping Protection of Arabidopsis CTC1.
Vaquero-Sedas MI, Vega-Palas MA. Vaquero-Sedas MI, et al. Int J Mol Sci. 2024 Jan 4;25(1):672. doi: 10.3390/ijms25010672. Int J Mol Sci. 2024. PMID: 38203842 Free PMC article. - Noncanonical functions of Ku may underlie essentiality in human cells.
Kelly RD, Parmar G, Bayat L, Maitland MER, Lajoie GA, Edgell DR, Schild-Poulter C. Kelly RD, et al. Sci Rep. 2023 Jul 27;13(1):12162. doi: 10.1038/s41598-023-39166-7. Sci Rep. 2023. PMID: 37500706 Free PMC article. - Telomeres and Their Neighbors.
Jenner LP, Peska V, Fulnečková J, Sýkorová E. Jenner LP, et al. Genes (Basel). 2022 Sep 16;13(9):1663. doi: 10.3390/genes13091663. Genes (Basel). 2022. PMID: 36140830 Free PMC article. Review. - Intra-epithelial non-canonical Activin A signaling safeguards prostate progenitor quiescence.
Cambuli F, Foletto V, Alaimo A, De Felice D, Gandolfi F, Palumbieri MD, Zaffagni M, Genovesi S, Lorenzoni M, Celotti M, Bertossio E, Mazzero G, Bertossi A, Bisio A, Berardinelli F, Antoccia A, Gaspari M, Barbareschi M, Fiorentino M, Shen MM, Loda M, Romanel A, Lunardi A. Cambuli F, et al. EMBO Rep. 2022 May 4;23(5):e54049. doi: 10.15252/embr.202154049. Epub 2022 Mar 7. EMBO Rep. 2022. PMID: 35253958 Free PMC article.
References
- Alexander P. and Mikulski, Z.B. (1961) Mouse lymphoma cells with different radiosensitivities. Nature, 192, 572–573. - PubMed
- Autexier C., and Greider, C.W. (1996) Telomerase and cancer: revisiting the telomere hypothesis. Trends Biochem. Sci., 21, 387–391. - PubMed
- Bianchi A. and de Lange, T. (1999) Ku binds telomeric DNA in vitro. J. Biol. Chem., 274, 21223–21227. - PubMed
- Blackburn E.H. (1991) Structure and function of telomeres. Nature, 350, 569–573. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous