Visualizing the spindle checkpoint in Drosophila spermatocytes - PubMed (original) (raw)
Visualizing the spindle checkpoint in Drosophila spermatocytes
E Rebollo et al. EMBO Rep. 2000 Jul.
Abstract
The spindle assembly checkpoint detects defects in spindle structure or in the alignment of the chromosomes on the metaphase plate and delays the onset of anaphase until defects are corrected. Thus far, the evidence regarding the presence of a spindle checkpoint during meiosis in male Drosophila has been indirect and contradictory. On the one hand, chromosomes without pairing partners do not prevent meiosis progression. On the other hand, some conserved components of the spindle checkpoint machinery are expressed in these cells and behave as their homologue proteins do in systems with an active spindle checkpoint. To establish whether the spindle checkpoint is active in Drosophila spermatocytes we have followed meiosis progression by time-lapse microscopy under conditions where the checkpoint is likely to be activated. We have found that the presence of a relatively high number of misaligned chromosomes or a severe disruption of the meiotic spindle results in a significant delay in the time of entry into anaphase. These observations provide the first direct evidence substantiating the activity of a meiotic spindle checkpoint in male Drosophila.
Figures
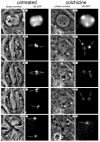
Fig. 1. Meiosis I progression in control and colchicine-treated wild-type males observed by time-lapse phase-contrast and fluorescence microscopy. Meiosis was followed from early prometaphase to the end of meiosis I at a rate of 20 frames/min using a combination of phase-contrast and fluorescence microscopy. The chromosomes were labelled with a His2–GFP fusion protein. Only five significant time points are shown in this figure. Control: time 0′ corresponds to late prophase. At this stage, the two centrosomes have migrated to opposite poles and organize large asters. The incipient meiotic spindle is outlined by associated phase-dark parafusorial membranes (asterisks) that contain numerous mitochondria (Rieder et al., 1994). A non-chromosomal phase-dark nuclear structure, which remains present throughout meiosis (McKee et al., 1998) can also be seen (arrowhead). At time 32′, the spermatocyte contains a fully formed, elongated spindle. Two bivalents can be seen stabilized at the metaphase plate in this focal plane (arrows). At time 37′, anaphase has just started. Two pairs of homologous chromosomes can be seen segregating from each other (double arrows). At time 41′, the chromosomes have reached the poles and are decondensing. Chromosome decondensation is first apparent midway through anaphase. At time 55′, the two daughter nuclei are formed (arrows) and the cytokinesis furrow begins to pinch the central spindle (white arrowheads). The parafusorial membranes are tightly associated with the spindle at this stage. Colchicine: time point 0′ corresponds to an early stage of chromatin condensation very similar to that shown by the control cell. The parafusorial membranes are not organized. At time point 31′, chromosome condensation has proceeded, but the chromosomes do not congress at a metaphase plate. At time point 51′ the bivalent that is within the focal plane can be seen to split into two homologous chromosomes (double arrow). Despite their separation, the two homologues do not segregate from each other to any noticeable distance. At time 60′, the chromosomes begin to decondense (arrows). At time point 74′, the nuclear envelope forms around the unsegregated chromosomes. Several clusters of parafusorial membranes can be seen scattered within this cell (asterisk). The lack of organization of these membranes throughout meiosis is fully consistent with a general failure in microtubule polymerization and provides an excellent internal control for the effect of colchicine in these cells.
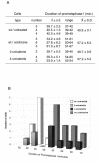
Fig. 2. Duration of prometaphase I under conditions that may trigger a spindle checkpoint. (A) Duration of prometaphase I was estimated as the interval spanning between the beginning of chromosome condensation and the onset of anaphase I. Four different conditions including wild-type untreated cells, wild-type cells treated with colchicine, and cells containing two [_C(3L);C(3R)_] or four [_C(2L);C(2R);C(3L);C(3R)_] univalent chromosomes were studied. Each line shows the number of cells followed in each single culture, together with the average (x), standard deviation (s.d.) and range of prometaphase length. The cumulative average of all the cultures from the same experiment and the corresponding standard deviation are also shown (X ± S.D.). Colchicine treatment of wild-type spermatocytes, as well as the presence of four univalents result in a delay of around 16 min, with respect to the control, while the presence of two univalents has no effect. (B) Graphic representation of the data presented in (A) showing the frequency distribution of the duration of prometaphase in the four experimental conditions studied.
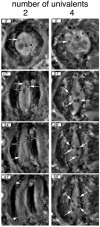
Fig. 3. Meiosis I progression in the presence of two [_C(3L);C(3R)_] and four [_C(2L);C(2R);C(3L);C(3R)_] univalent chromosomes observed by time-lapse phase-contrast microscopy. Meiosis was followed from late prometaphase to the end of meiosis I at a rate of 20 frames/min. Only four significant time points are shown in this figure. Two univalents: time point 0′ corresponds to early prometaphase. One univalent (white arrow) and one bivalent (black arrow) can be observed at this focal plane. At time 17′, the bivalents (black arrow) are stabilized at the metaphase plate while the two univalents (white arrows) oscillate between the two poles. At time 34′, anaphase has started. Two pairs of segregating homologues (double arrows) and one univalent located near one pole (white arrow) can be observed. At time point 41′, the chromosomes have reached the poles and are fully decondensed (arrowheads). The chromosomes were also followed by fluorescence microscopy to detect the His2–GFP fusion (not shown). Four univalents: time point 0′ corresponds to the beginning of prometaphase. One univalent (white arrow) can be observed at this focal plane. At time points 21′, 28′ and 55′, the four univalents (white arrows), and the major bivalent, the X/Y pair (black arrow), remain condensed. The X and Y chromosomes do not segregate during this period. Anaphase onset in this cell started at time point 59′ (not shown), a delay of >20 min over the cell that carries two univalents.
Similar articles
- Inhibition of Aurora kinases perturbs chromosome alignment and spindle checkpoint signaling in rat spermatocytes.
Wang Y, Toppari J, Parvinen M, Kallio MJ. Wang Y, et al. Exp Cell Res. 2006 Nov 1;312(18):3459-70. doi: 10.1016/j.yexcr.2006.04.026. Epub 2006 Aug 12. Exp Cell Res. 2006. PMID: 16962097 - Spindle self-organization and cytokinesis during male meiosis in asterless mutants of Drosophila melanogaster.
Bonaccorsi S, Giansanti MG, Gatti M. Bonaccorsi S, et al. J Cell Biol. 1998 Aug 10;142(3):751-61. doi: 10.1083/jcb.142.3.751. J Cell Biol. 1998. PMID: 9700163 Free PMC article. - What Drosophila spermatocytes tell us about the mechanisms underlying cytokinesis.
Giansanti MG, Fuller MT. Giansanti MG, et al. Cytoskeleton (Hoboken). 2012 Nov;69(11):869-81. doi: 10.1002/cm.21063. Epub 2012 Sep 21. Cytoskeleton (Hoboken). 2012. PMID: 22927345 Free PMC article. Review. - Microtubule and Actin Cytoskeletal Dynamics in Male Meiotic Cells of Drosophila melanogaster.
Frappaolo A, Piergentili R, Giansanti MG. Frappaolo A, et al. Cells. 2022 Feb 16;11(4):695. doi: 10.3390/cells11040695. Cells. 2022. PMID: 35203341 Free PMC article. Review.
Cited by
- The Drosophila kinesin-like protein KLP67A is essential for mitotic and male meiotic spindle assembly.
Gandhi R, Bonaccorsi S, Wentworth D, Doxsey S, Gatti M, Pereira A. Gandhi R, et al. Mol Biol Cell. 2004 Jan;15(1):121-31. doi: 10.1091/mbc.e03-05-0342. Epub 2003 Sep 17. Mol Biol Cell. 2004. PMID: 13679514 Free PMC article. - The analysis of mutant alleles of different strength reveals multiple functions of topoisomerase 2 in regulation of Drosophila chromosome structure.
Mengoli V, Bucciarelli E, Lattao R, Piergentili R, Gatti M, Bonaccorsi S. Mengoli V, et al. PLoS Genet. 2014 Oct 23;10(10):e1004739. doi: 10.1371/journal.pgen.1004739. eCollection 2014 Oct. PLoS Genet. 2014. PMID: 25340516 Free PMC article. - The Janus soul of centrosomes: a paradoxical role in disease?
Nano M, Basto R. Nano M, et al. Chromosome Res. 2016 Jan;24(1):127-44. doi: 10.1007/s10577-015-9507-3. Chromosome Res. 2016. PMID: 26643310 Review. - Mutations in orbit/mast reveal that the central spindle is comprised of two microtubule populations, those that initiate cleavage and those that propagate furrow ingression.
Inoue YH, Savoian MS, Suzuki T, Máthé E, Yamamoto MT, Glover DM. Inoue YH, et al. J Cell Biol. 2004 Jul 5;166(1):49-60. doi: 10.1083/jcb.200402052. J Cell Biol. 2004. PMID: 15240569 Free PMC article. - Cdc37 is essential for chromosome segregation and cytokinesis in higher eukaryotes.
Lange BM, Rebollo E, Herold A, González C. Lange BM, et al. EMBO J. 2002 Oct 15;21(20):5364-74. doi: 10.1093/emboj/cdf531. EMBO J. 2002. PMID: 12374737 Free PMC article.
References
- Ashburner M. (1989) Drosophila: A Laboratory Handbook. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Basu J., Logarinho, E., Herrmann, S., Bousbaa, H., Li, Z., Chan, G.T.K., Yen, T.J., Sunkel, C.E. and Goldberg, M.L. (1998) Localization of the Drosophila checkpoint control protein Bub3 to the kinetochore requires Bub1 but not Zw10 or Rod. Chromosoma, 107, 376–385. - PubMed
- Church K. and Lin, H.-P.P. (1985) Kinetochore microtubules and chromosome movement during prometaphase in Drosophila melanogaster spermatocytes studied in life and with the electron microscope. Chromosoma, 92, 273–282. - PubMed
- Church K. and Lin, H.-P.P. (1988) Drosophila: A model for the study of aneuplopidy. In Aneuploidy, part B. Alan R. Liss, Inc., pp. 227–255.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases