Enhanced expression of the alpha 7 beta 1 integrin reduces muscular dystrophy and restores viability in dystrophic mice - PubMed (original) (raw)
Enhanced expression of the alpha 7 beta 1 integrin reduces muscular dystrophy and restores viability in dystrophic mice
D J Burkin et al. J Cell Biol. 2001.
Abstract
Muscle fibers attach to laminin in the basal lamina using two distinct mechanisms: the dystrophin glycoprotein complex and the alpha 7 beta 1 integrin. Defects in these linkage systems result in Duchenne muscular dystrophy (DMD), alpha 2 laminin congenital muscular dystrophy, sarcoglycan-related muscular dystrophy, and alpha 7 integrin congenital muscular dystrophy. Therefore, the molecular continuity between the extracellular matrix and cell cytoskeleton is essential for the structural and functional integrity of skeletal muscle. To test whether the alpha 7 beta 1 integrin can compensate for the absence of dystrophin, we expressed the rat alpha 7 chain in mdx/utr(-/-) mice that lack both dystrophin and utrophin. These mice develop a severe muscular dystrophy highly akin to that in DMD, and they also die prematurely. Using the muscle creatine kinase promoter, expression of the alpha 7BX2 integrin chain was increased 2.0-2.3-fold in mdx/utr(-/-) mice. Concomitant with the increase in the alpha 7 chain, its heterodimeric partner, beta 1D, was also increased in the transgenic animals. Transgenic expression of the alpha 7BX2 chain in the mdx/utr(-/-) mice extended their longevity by threefold, reduced kyphosis and the development of muscle disease, and maintained mobility and the structure of the neuromuscular junction. Thus, bolstering alpha 7 beta 1 integrin-mediated association of muscle cells with the extracellular matrix alleviates many of the symptoms of disease observed in mdx/utr(-/-) mice and compensates for the absence of the dystrophin- and utrophin-mediated linkage systems. This suggests that enhanced expression of the alpha 7 beta 1 integrin may provide a novel approach to treat DMD and other muscle diseases that arise due to defects in the dystrophin glycoprotein complex. A video that contrasts kyphosis, gait, joint contractures, and mobility in mdx/utr(-/-) and alpha 7BX2-mdx/utr(-/-) mice can be accessed at http://www.jcb.org/cgi/content/full/152/6/1207.
Figures
Figure 1
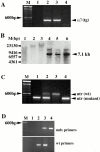
Genotyping transgenic α7BX2-mdx/utr−/− mice. (A) The α7BX2 transgene (tg) was detected by PCR using primers that amplify between the MCK promoter and the α7 cDNA sequence. Lanes 2 and 3 are positive for the MCK-α7BX2 transgene. (B) Southern blot analysis using a rat α7-specific probe of EcoRI- and KpnI-digested genomic DNA. The 7.1-kb band corresponding to the rat transgene construct is detected in lanes 4–6. A higher 14.2-kb transgene dimer was also detected. Samples in these lanes are from α_7BX2-mdx/utr−/−_ mice. DNA in lanes 1–3 are from nontransgenic mice. (C) Determining the status of the utrophin gene by PCR. Only mutant utr alleles are detected in lanes 1 and 4, identifying utr−/− mice. One wild-type (wt) and one mutant allele are amplified in lane 2, identifying a utr +/− mouse. Lane 3 is wild-type at both utr loci. (D) Determining the status of the dystrophin gene by PCR. The mdx primer set detects the point mutation in the dystrophin gene, whereas the wt primers detect only the wild-type allele. Mouse 2 is wild-type at the dystrophin locus, mouse 3 is heterozygous (mdx/+), and mouse 4 is mdx. Lane 1 contains no DNA.
Figure 4
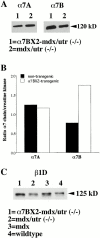
Transgenic expression of α7BX2 increases the amount of β1D in hindlimb muscle. (A) Western blot showing more α7B is detected in transgenic mice compared with nontransgenic mice, whereas α7A is constant. (B) The blots were reprobed with anti-creatine kinase antibody. The creatine kinase levels were used to normalize the amounts of α7A and α7B proteins in each sample. The levels of α7A/creatinine kinase in both transgenic and nontransgenic mice remained constant. In contrast, the α7B/creatinine kinase ratio is 2.3-fold higher in the α7BX2 transgenic mice compared with the nontransgenic animal. Comparisons relative to total protein stained with Ponceau S indicate a 2.0-fold increase. (C) β1D integrin from 8 wk hindlimb muscle. Less β1D is detected in mdx/utr−/− mice compared with α7BX2-mdx/utr−/− mice. Compared with total protein, an increase of approximately 1.5-fold more β1D was detected in the transgenic versus nontransgenic mice. Similar results were obtained in duplicate experiments.
Figure 2
Expression of the rat α7 protein in mouse muscle. Immunofluorescence analysis of hindlimb cryosections using monoclonal antibodies against the rat α7 integrin chain, dystrophin, and utrophin. AChRs were stained with rhodamine-labeled α-bungarotoxin to identify NMJs, the sites of normal utrophin localization. The rat α7 protein is only detected in transgenic mice and localizes to the membrane of muscle fibers. The lack of dystrophin and utrophin in both transgenic and nontransgenic mdx/utr−/− mice confirms their genotypes. The fluorescent specks seen in the mdx/utr−/− muscle stained with mouse antidystrophin, antiutrophin, and anti-α7 integrin antibodies are also evident in the absence of primary antibody and are due to residual staining with secondary anti–mouse antibody.
Figure 3
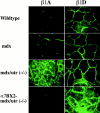
Immunofluores-cence of β1 integrin isoforms in the hindlimb of 8-wk-old wild-type, mdx, mdx/utr−/−, and α7BX2-mdx/utr−/− mice. β1A integrin is elevated in muscle fibers of mdx/utr−/− mice compared with wild-type and mdx animals. In contrast, β1A levels are normal in α7BX2-mdx/utr−/− mice. Compared with wild-type, an increase in β1D is detected in both mdx and mdx/utr−/− muscle. α7BX2-mdx/utr−/− mice show an additional increase in β1D compared with both mdx and mdx/utr−/− mice.
Figure 5
Kaplan-Meier survival curves of 43 α7BX2-mdx/utr−/− and 84 mdx/utr−/− mice. Wilcoxon and log-rank tests show that the α_7BX2-mdx/utr−/−_ and mdx/utr−/− mice populations have distinct survival curves (P < 0.001). The α_7BX2-mdx/utr−/−_ mice survive threefold longer than nontransgenic mdx/utr−/− mice with a median life expectancy of 38 wk. In contrast, nontransgenic mdx/utr−/− mice have a median life expectancy of 12 wk (95% confidence intervals are indicated by shading).
Figure 6
Weight gain versus survival in five representative mdx/utr−/− mice and α_7BX2-mdx/utr−/−_ mice. The majority of nontransgenic mdx/utr−/− mice undergo a crisis at 5–10 wk of age that results in a sudden loss of weight and premature death. Most transgenic mdx/utr−/− mice live longer and maintain weight. Eventually these too will go through a crisis that results in weight loss. The mean life span of the mdx/utr−/− mice illustrated here is 10.4 wk; the mean life span of the α7BX2-mdx/utr−/− mice illustrated here is 41.8 wk.
Figure 8
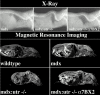
X-ray and MRI of normal and dystrophic mice. Top three panels, the severe spinal curvature (kyphosis) and constriction of the rib cage in mdx/utr−/− mice are largely reduced in the α7BX2 transgenic animals; bottom four panels, MRI of midsagittal sections reveals that kyphosis and reduction of pulmonary volume in mdx/utr−/− mice are largely alleviated in transgenic mice.
Figure 7
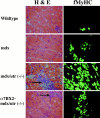
Histology of hindlimbs from 10-wk-old wild-type, mdx, mdx/utr−/−, and α_7BX2-mdx/utr−/−_ mice. Hematoxylin and eosin (H & E) staining reveals abundant central nuclei in mdx, mdx/utr−/−, and α7BX2-mdx/utr−/− mice. Mononuclear cell infiltration (arrows) and expression of fMyHC are extensive in the mdx/utr−/− mice, but are reduced in the α7BX2-mdx/utr−/− transgenic animals, indicating less degeneration and more stable regeneration in these mice.
Figure 9
Severe spinal curvature (kyphosis) and hindlimb clasping (joint contractures) are largely reduced in mice expressing the rat α7BX2 transgene.
Figure 10
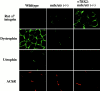
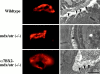
Structure of the NMJ in 5-wk-old wild-type, mdx/utr−/−, and α7BX2-mdx/utr−/− mice. (Left) En face views of AChRs in the postsynaptic membrane detected with rhodamine-labeled α-bungarotoxin. In wild-type mice, the junctions appear continuous, folded, and uninterrupted. In mdx/utr−/− mice, the distribution of AChRs is discontinuous and organized into discrete boutons. The organization of the postsynaptic membrane in α7BX2-mdx/utr−/− transgenic mice has a more continuous (normal) pattern. (Right) Ultrastructural changes in the NMJ. The postsynatic membrane of wild-type mice is highly folded (arrowheads). In contrast, mdx/utr−/− mice have little or no membrane folding. Expression of the α7BX2 transgene in mdx/utr−/− mice results in a postsynaptic membrane with partially restored folding (arrowheads). Bar, 1 μm.
Similar articles
- Differential effects of dystrophin and utrophin gene transfer in immunocompetent muscular dystrophy (mdx) mice.
Ebihara S, Guibinga GH, Gilbert R, Nalbantoglu J, Massie B, Karpati G, Petrof BJ. Ebihara S, et al. Physiol Genomics. 2000 Sep 8;3(3):133-44. doi: 10.1152/physiolgenomics.2000.3.3.133. Physiol Genomics. 2000. PMID: 11015608 - Sarcospan integration into laminin-binding adhesion complexes that ameliorate muscular dystrophy requires utrophin and α7 integrin.
Marshall JL, Oh J, Chou E, Lee JA, Holmberg J, Burkin DJ, Crosbie-Watson RH. Marshall JL, et al. Hum Mol Genet. 2015 Apr 1;24(7):2011-22. doi: 10.1093/hmg/ddu615. Epub 2014 Dec 11. Hum Mol Genet. 2015. PMID: 25504048 Free PMC article. - Overexpression of the cytotoxic T cell GalNAc transferase in skeletal muscle inhibits muscular dystrophy in mdx mice.
Nguyen HH, Jayasinha V, Xia B, Hoyte K, Martin PT. Nguyen HH, et al. Proc Natl Acad Sci U S A. 2002 Apr 16;99(8):5616-21. doi: 10.1073/pnas.082613599. Proc Natl Acad Sci U S A. 2002. PMID: 11960016 Free PMC article. - Dystrophin and utrophin: genetic analyses of their role in skeletal muscle.
Rafael JA, Brown SC. Rafael JA, et al. Microsc Res Tech. 2000 Feb 1-15;48(3-4):155-66. doi: 10.1002/(SICI)1097-0029(20000201/15)48:3/4<155::AID-JEMT4>3.0.CO;2-0. Microsc Res Tech. 2000. PMID: 10679963 Review. - An attempt of gene therapy in Duchenne muscular dystrophy: overexpression of utrophin in transgenic mdx mice.
Gillis JM. Gillis JM. Acta Neurol Belg. 2000 Sep;100(3):146-50. Acta Neurol Belg. 2000. PMID: 11098286 Review.
Cited by
- β1-integrins signaling and mammary tumor progression in transgenic mouse models: implications for human breast cancer.
Lahlou H, Muller WJ. Lahlou H, et al. Breast Cancer Res. 2011;13(6):229. doi: 10.1186/bcr2905. Epub 2011 Nov 30. Breast Cancer Res. 2011. PMID: 22264244 Free PMC article. Review. - Transgenic overexpression of α7 integrin in smooth muscle attenuates allergen-induced airway inflammation in a murine model of asthma.
Ba MA, Aiyuk A, Hernández K, Evasovic JM, Wuebbles RD, Burkin DJ, Singer CA. Ba MA, et al. FASEB Bioadv. 2022 Sep 12;4(11):724-740. doi: 10.1096/fba.2022-00050. eCollection 2022 Nov. FASEB Bioadv. 2022. PMID: 36349295 Free PMC article. - Role of extracellular matrix proteins and their receptors in the development of the vertebrate neuromuscular junction.
Singhal N, Martin PT. Singhal N, et al. Dev Neurobiol. 2011 Nov;71(11):982-1005. doi: 10.1002/dneu.20953. Dev Neurobiol. 2011. PMID: 21766463 Free PMC article. Review. - Expression of the dystrophin isoform Dp116 preserves functional muscle mass and extends lifespan without preventing dystrophy in severely dystrophic mice.
Judge LM, Arnett AL, Banks GB, Chamberlain JS. Judge LM, et al. Hum Mol Genet. 2011 Dec 15;20(24):4978-90. doi: 10.1093/hmg/ddr433. Epub 2011 Sep 23. Hum Mol Genet. 2011. PMID: 21949353 Free PMC article. - Vascular delivery of rAAVrh74.MCK.GALGT2 to the gastrocnemius muscle of the rhesus macaque stimulates the expression of dystrophin and laminin α2 surrogates.
Chicoine LG, Rodino-Klapac LR, Shao G, Xu R, Bremer WG, Camboni M, Golden B, Montgomery CL, Shontz K, Heller KN, Griffin DA, Lewis S, Coley BD, Walker CM, Clark KR, Sahenk Z, Mendell JR, Martin PT. Chicoine LG, et al. Mol Ther. 2014 Apr;22(4):713-24. doi: 10.1038/mt.2013.246. Epub 2013 Oct 22. Mol Ther. 2014. PMID: 24145553 Free PMC article.
References
- Amalfitano A., Chamberlain J.S. The mdx-amplification-resistant mutation system assay, a simple and rapid polymerase chain reaction-based detection of the mdx allele. Muscle Nerve. 1996;19:1549–1553. - PubMed
- Belkin A.M., Zhidkova N.I., Balzac F., Altruda F., Tomatis D., Maier A., Tarone G., Koteliansky V.E., Burridge K. Beta 1D integrin displaces the beta 1A isoform in striated muscleslocalization at junctional structures and signaling potential in nonmuscle cells. J. Cell Biol. 1996;132:211–216. - PMC - PubMed
- Bertorini T.E., Bhattacharya S.K., Palmieri G.M., Chesney C.M., Pifer D., Baker B. Muscle calcium and magnesium content in Duchenne muscular dystrophy. Neurology. 1982;32:1088–1092. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases