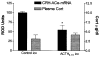Corticotropin (ACTH) acts directly on amygdala neurons to down-regulate corticotropin-releasing hormone gene expression - PubMed (original) (raw)
Corticotropin (ACTH) acts directly on amygdala neurons to down-regulate corticotropin-releasing hormone gene expression
K L Brunson et al. Ann Neurol. 2001 Mar.
Abstract
The hormone corticotropin (ACTH) is employed as therapy for diverse neurological disorders, but the mechanisms for its efficacy remain unknown. ACTH promotes the release of adrenal steroids (glucocorticoids), and most ACTH effects on the central nervous system (CNS) have been attributed to activation of glucocorticoid receptors. However, in several human disorders, ACTH has therapeutic actions that differ qualitatively or quantitatively from those of steroids. This study tested the hypothesis that ACTH directly influences limbic neurons via the recently characterized melanocortin receptors and focused on the effects of ACTH on the expression of corticotropin-releasing hormone (CRH), a neuropeptide involved in neuroimmune functions and in certain developmental seizures. The results demonstrated that ACTH potently reduced CRH expression in amygdala neurons. This down-regulation was not abolished by experimental elimination of steroids or by blocking their receptors and was reproduced by a centrally administered ACTH fragment that does not promote steroid release. Importantly, selective blocking of melanocortin receptors prevented ACTH-induced down-regulation of CRH expression. Taken together, these data indicate that ACTH activates central melanocortin receptors to modulate CRH gene expression in amygdala, supporting the notion that direct, steroid-independent actions of ACTH may account for some of its established clinical effects on the CNS.
Figures
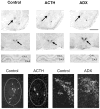
Fig 1
Differential regulation of CRH mRNA levels in ACe, hippocampus, and hypothalamic PVN by ACTH and adrenal steroids. Photomicrographs of coronal brain sections subjected to ISH for CRH mRNA. CRH mRNA expression in ACe (arrows, top row), PVN (arrows, second row), and hippocampus (third row) are shown. ACTH treatment decreased CRH mRNA signal over ACe. Adrenalectomy (ADX), by increasing endogenous ACTH levels, led to a similar reduction of CRH gene expression in ACe (top row). In contrast to the ACe, ADX resulted in up-regulation of CRH mRNA levels in PVN (middle row) and reduced CRH mRNA signal in hippocampus (third row) compared to control sections. The bottom row depicts representative darkfield photomicrographs of ACe (encircled region in left two panels) and PVN (right two panels). A marked reduction in the number of CRH-expressing cells is evident in ACTH-treated animals compared to controls. In PVN, increased CRH mRNA signal is visible in sections from an ADX animal. Scale bar = 1,500 μm in top two rows, 90 μm in third row, 200 μm in bottom row. Asterisks = basolateral nucleus; mpd, mpv = medial dorsal and medial ventral parvicellular cell groups of PVN, respectively.
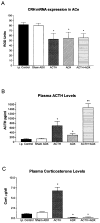
Fig 2
Effects of ACTH on CRH mRNA levels in ACe. (A) Semiquantitative analysis of CRH mRNA expression in ACe of immature rats subjected to selective hormone level manipulations. Signal was analyzed over ACe following ISH as discussed in Materials and Methods. (B) CRH mRNA levels in ACe correlated inversely with plasma ACTH. Thus, ACTH administration (ACTH i.p.) or augmentation of endogenous ACTH (ADX) or both (ACTH i.p. + ADX) significantly reduced CRH mRNA levels in ACe compared to both the i.p. vehicle-injected and the sham-adrenalectomy, vehicle-injected control groups. No correlation between plasma corticosterone levels and ACe-CRH mRNA was observed (C). *Significant difference from control and sham ADX; **significant difference from all other groups (p < 0.05). Values depict means ± SEM. ADX = adrenalectomy.
Fig 3
CRH mRNA expression in ACe is down-regulated by centrally administered ACTH analog that is devoid of glucocorticoid secretion. Semiquantitative analysis (see Materials and Methods) of CRH mRNA signal in sections of animals receiving an i.c.v. infusion of ACTH4–10, an analog binding melanocortin receptors but not stimulating corticosterone (Cort). Although infusion of ACTH4–10 did not induce Cort secretion (hatched bars, scale on right), this analog reduced ACe-CRH mRNA levels significantly (solid bars, left scale). Values are means ± SEM; *p < 0.05.
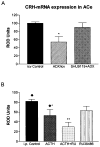
Fig 4
Effects of ACTH on CRH mRNA expression in ACe require activation of MC4-Rs but not of glucocorticoid receptors. (A) High endogenous plasma ACTH levels were achieved by ADX, significantly reducing CRH mRNA levels (single asterisks). Administration of SHU9119, however, abolished ACTH-induced reduction of CRH mRNA. (B) In contrast, coadministered RU 38486 failed to block the effects of ACTH (i.p.) on CRH mRNA expression in ACe. In fact, CRH mRNA levels in ACTH+ RU 38486 treated rats were significantly lower from those given ACTH alone, as denoted by double asterisks. Values denote means ± SEM of values achieved using semiquantitative analysis (see Materials and Methods). i.c.v. Controls and i.p. controls received vehicle via the noted routes; ADX/icv were adrenalectomized and given i.c.v. vehicle. Diamonds over the i.p. control and ACTH groups indicate that they correspond to groups shown in Fig 2A.
Fig 5
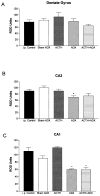
Differential regulation of CRH mRNA expression in hippocampal regions of immature rats by glucocorticoids and ACTH. Semiquantitative analysis of signal over dentate gyrus (A), CA3 (B), and CA1 (C) was performed after ISH, as described in Materials and Methods. CRH mRNA levels in CA1 (the region rich in steroid receptors in the immature rat) were reduced by elimination of GCs (ADX), regardless of ACTH levels (C). CRH mRNA expression in other hippocampal regions was not appreciably altered by these hormonal manipulations.*Significant difference from i.p. vehicle-injected control and sham-ADX animals given i.p. vehicle (p < 0.05). Values are means ± SEM. ADX = adrenalectomy.
Similar articles
- ACTH treatment of infantile spasms: mechanisms of its effects in modulation of neuronal excitability.
Brunson KL, Avishai-Eliner S, Baram TZ. Brunson KL, et al. Int Rev Neurobiol. 2002;49:185-97. doi: 10.1016/s0074-7742(02)49013-7. Int Rev Neurobiol. 2002. PMID: 12040892 Free PMC article. Review. - Systemic adrenocorticotropic hormone administration down-regulates the expression of corticotropin-releasing hormone (CRH) and CRH-binding protein in infant rat hippocampus.
Wang W, Murphy B, Dow KE, David Andrew R, Fraser DD. Wang W, et al. Pediatr Res. 2004 Apr;55(4):604-10. doi: 10.1203/01.PDR.0000112105.33521.DC. Epub 2004 Jan 7. Pediatr Res. 2004. PMID: 14711894 - The regulation of adrenocorticotrophic hormone receptor by corticotropin-releasing hormone in human fetal adrenal definitive/transitional zone cells.
Rehman KS, Sirianni R, Parker CR Jr, Rainey WE, Carr BR. Rehman KS, et al. Reprod Sci. 2007 Sep;14(6):578-87. doi: 10.1177/1933719107307908. Reprod Sci. 2007. PMID: 17959886 - Corticotropin-releasing hormone (CRH) downregulates the function of its receptor (CRF1) and induces CRF1 expression in hippocampal and cortical regions of the immature rat brain.
Brunson KL, Grigoriadis DE, Lorang MT, Baram TZ. Brunson KL, et al. Exp Neurol. 2002 Jul;176(1):75-86. doi: 10.1006/exnr.2002.7937. Exp Neurol. 2002. PMID: 12093084 Free PMC article. - Glucocorticoid regulation of peptide genes in neuroendocrine CRH neurons: a complexity beyond negative feedback.
Watts AG. Watts AG. Front Neuroendocrinol. 2005 Oct-Dec;26(3-4):109-30. doi: 10.1016/j.yfrne.2005.09.001. Epub 2005 Nov 10. Front Neuroendocrinol. 2005. PMID: 16289311 Review.
Cited by
- Infantile Spasms: An Update on Pre-Clinical Models and EEG Mechanisms.
Janicot R, Shao LR, Stafstrom CE. Janicot R, et al. Children (Basel). 2020 Jan 6;7(1):5. doi: 10.3390/children7010005. Children (Basel). 2020. PMID: 31935804 Free PMC article. Review. - Modeling epileptic spasms during infancy: Are we heading for the treatment yet?
Velíšek L, Velíšková J. Velíšek L, et al. Pharmacol Ther. 2020 Aug;212:107578. doi: 10.1016/j.pharmthera.2020.107578. Epub 2020 May 15. Pharmacol Ther. 2020. PMID: 32417271 Free PMC article. Review. - Hippocampal neuroplasticity induced by early-life stress: functional and molecular aspects.
Fenoglio KA, Brunson KL, Baram TZ. Fenoglio KA, et al. Front Neuroendocrinol. 2006 Jul;27(2):180-92. doi: 10.1016/j.yfrne.2006.02.001. Epub 2006 Apr 17. Front Neuroendocrinol. 2006. PMID: 16603235 Free PMC article. Review. - Neuropeptides as targets for the development of anticonvulsant drugs.
Clynen E, Swijsen A, Raijmakers M, Hoogland G, Rigo JM. Clynen E, et al. Mol Neurobiol. 2014 Oct;50(2):626-46. doi: 10.1007/s12035-014-8669-x. Epub 2014 Apr 6. Mol Neurobiol. 2014. PMID: 24705860 Free PMC article. Review. - Infantile spasms: a critical review of emerging animal models.
Stafstrom CE. Stafstrom CE. Epilepsy Curr. 2009 May-Jun;9(3):75-81. doi: 10.1111/j.1535-7511.2009.01299.x. Epilepsy Curr. 2009. PMID: 19471616 Free PMC article.
References
- Pranzatelli MR. On the molecular mechanism of adrenocorticotrophic hormone in the CNS: neurotransmitters and receptors. Exp Neurol. 1994;125:142–161. - PubMed
- Pranzatelli MR, Huang YY, Tate E, et al. Monoaminergic effects of high-dose corticotropin in corticotropin-responsive pediatric opsoclonus-myoclonus. Mov Disord. 1998;13:522–528. - PubMed
- Snead OC, III, Benton JW, Hosey LC, et al. Treatment of infantile spasms with high-dose ACTH: efficacy and plasma levels of ACTH and prednisone. Neurology. 1989;39:1027–1031. - PubMed
- Riikonen R. Infantile spasms: some new theoretical aspects. Epilepsia. 1983;24:159–168. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R41 HD34975/HD/NICHD NIH HHS/United States
- R01 NS028912-07A2S1/NS/NINDS NIH HHS/United States
- R01 NS28912/NS/NINDS NIH HHS/United States
- R01 NS039307-04/NS/NINDS NIH HHS/United States
- R01 NS028912-06/NS/NINDS NIH HHS/United States
- R01 NS028912-07A2/NS/NINDS NIH HHS/United States
- R01 NS028912/NS/NINDS NIH HHS/United States
- R01 NS039307/NS/NINDS NIH HHS/United States