The PACT domain, a conserved centrosomal targeting motif in the coiled-coil proteins AKAP450 and pericentrin - PubMed (original) (raw)
The PACT domain, a conserved centrosomal targeting motif in the coiled-coil proteins AKAP450 and pericentrin
A K Gillingham et al. EMBO Rep. 2000 Dec.
Abstract
AKAP450 (also known as AKAP350, CG-NAP or Hyperion) and pericentrin are large coiled-coil proteins found in mammalian centrosomes that serve to recruit structural and regulatory components including dynein and protein kinase A. We find that these proteins share a well conserved 90 amino acid domain near their C-termini that is also found in coiled-coil proteins of unknown function from Drosophila and fission yeast. Fusion of the C-terminal region from either protein to a reporter protein confers a centrosomal localization, and overexpression of the domain from AKAP450 displaces endogenous pericentrin, suggesting recruitment to a shared site. When isolated from transfected cells the C-terminal domain of AKAP450 was associated with calmodulin, suggesting that this protein could contribute to centrosome assembly.
Figures
Fig. 1. A conserved region at the C-terminus of a family of large coiled-coil proteins. (A) Structure of the human centrosomal proteins AKAP450 and pericentrin, and of proteins encoded by open reading frames from the indicated organisms. The related region is shown in black, and regions predicted to be predominantly coiled-coil are shown in grey. Pericentrin was originally cloned from mouse, and the human pericentrin homologue (kendrin) appears larger, but in fact the 3′ untranslated region of the original mouse cDNA ends with a sequence encoding a homologue of a known protein, suggesting it is a fusion of two cDNAs formed during cloning (Doxsey et al., 1994). (B) Alignment of the C-terminal sequences of the proteins in (A). Residues identical (black) or related (grey) in two or more of the sequences are shown, and the two particularly well conserved sections are underlined.
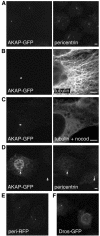
Fig. 2. The C-terminal regions of AKAP450 and pericentrin confer targeting to the centrosome. (A–F) Transfected COS cells expressing either GFP or RFP fused to the C-terminal regions of AKAP450 (C-terminal 266 residues, ‘AKAP’), pericentrin (241 residues, ‘peri’) or Drosophila CG6735 (226 residues, ‘Dros’), respectively. Cells were stained with antibodies against the indicated proteins, and nocodazole treatment was at 5 µM for 90 min. Scale bars, 5 µm.
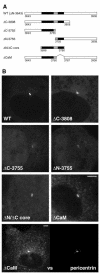
Fig. 3. At least two parts of the conserved region of the AKAP450 C-terminus contribute to centrosomal targeting. (A) The parts of the C-terminal region of AKAP450 fused to GFP in the indicated constructs. The residue numbers at the beginning and end of each construct are shown, with residue 3908 being the C-terminus of the whole protein. The two well conserved sections underlined in Figure 1B are shown in black. (B) COS cells expressing the six GFP fusions shown in (A). Cells were photographed using identical settings, and the perinuclear spots colocalized with endogenous pericentrin (not shown). In addition the lowest panel shows double labelling of endogenous pericentrin and ΔCaM at high levels of expression, the latter showing centrosomal staining and also a punctate pattern in the cytosol. Scale bars, 5 µm.
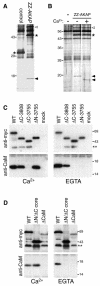
Fig. 4. The C-terminal region of AKAP450 coprecipitates from cells in a complex with calmodulin. (A) Silver-stained gel of proteins isolated from COS cells (two 10 cm dishes per lane) expressing protein A fusions to either the C-terminal residues of AKAP450 or the C-terminal 134 residues of golgin-84 (control). Protein A fusions are indicated by asterisks, the coprecipitating bands by arrowheads. An additional band (open arrowhead) was seen at variable levels and identified as mitochondrial hsp60. We have found heat shock proteins associating with several protein A fusions, presumably reflecting binding to a partially folded proportion of overexpressed chimera. (B) As (A) except that the control was mock-transfected cells (–), and the samples in SDS buffer were divided in two and adjusted to 10 mM Ca2+ or 10 mM EGTA prior to electrophoresis. (C and D) Immunoblots of anti-GFP immunoprecipitates isolated from cells expressing GFP fused to parts of AKAP450 schematized in Figure 3A, or mock transfected (mock). Precipitation was in the presence of 10 mM Ca2+ or EGTA, and the blots were probed for calmodulin (CaM) or the Myc-tag present in the GFP fusion. A minor band corresponding to a smaller GFP fusion (**) was not visible in total cell samples and presumably reflects proteolysis post-lysis.
Similar articles
- Centrosomal proteins CG-NAP and kendrin provide microtubule nucleation sites by anchoring gamma-tubulin ring complex.
Takahashi M, Yamagiwa A, Nishimura T, Mukai H, Ono Y. Takahashi M, et al. Mol Biol Cell. 2002 Sep;13(9):3235-45. doi: 10.1091/mbc.e02-02-0112. Mol Biol Cell. 2002. PMID: 12221128 Free PMC article. - Centrosomal anchoring of the protein kinase CK1delta mediated by attachment to the large, coiled-coil scaffolding protein CG-NAP/AKAP450.
Sillibourne JE, Milne DM, Takahashi M, Ono Y, Meek DW. Sillibourne JE, et al. J Mol Biol. 2002 Sep 27;322(4):785-97. doi: 10.1016/s0022-2836(02)00857-4. J Mol Biol. 2002. PMID: 12270714 - Cloning and characterization of a cDNA encoding an A-kinase anchoring protein located in the centrosome, AKAP450.
Witczak O, Skålhegg BS, Keryer G, Bornens M, Taskén K, Jahnsen T, Orstavik S. Witczak O, et al. EMBO J. 1999 Apr 1;18(7):1858-68. doi: 10.1093/emboj/18.7.1858. EMBO J. 1999. PMID: 10202149 Free PMC article. - AKAP signaling complexes at the cytoskeleton.
Diviani D, Scott JD. Diviani D, et al. J Cell Sci. 2001 Apr;114(Pt 8):1431-7. doi: 10.1242/jcs.114.8.1431. J Cell Sci. 2001. PMID: 11282019 Review. - CG-NAP/Kinase Interactions Fine-Tune T Cell Functions.
Verma NK, Chalasani MLS, Scott JD, Kelleher D. Verma NK, et al. Front Immunol. 2019 Nov 12;10:2642. doi: 10.3389/fimmu.2019.02642. eCollection 2019. Front Immunol. 2019. PMID: 31781123 Free PMC article. Review.
Cited by
- Chemical tools for dissecting cell division.
Chen GY, Lampson MA. Chen GY, et al. Nat Chem Biol. 2021 Jun;17(6):632-640. doi: 10.1038/s41589-021-00798-3. Epub 2021 May 25. Nat Chem Biol. 2021. PMID: 34035515 Free PMC article. Review. - Centrosomal AKAP350 and CIP4 act in concert to define the polarized localization of the centrosome and Golgi in migratory cells.
Tonucci FM, Hidalgo F, Ferretti A, Almada E, Favre C, Goldenring JR, Kaverina I, Kierbel A, Larocca MC. Tonucci FM, et al. J Cell Sci. 2015 Sep 1;128(17):3277-89. doi: 10.1242/jcs.170878. Epub 2015 Jul 24. J Cell Sci. 2015. PMID: 26208639 Free PMC article. - Par6γ is at the mother centriole and controls centrosomal protein composition through a Par6α-dependent pathway.
Dormoy V, Tormanen K, Sütterlin C. Dormoy V, et al. J Cell Sci. 2013 Feb 1;126(Pt 3):860-70. doi: 10.1242/jcs.121186. Epub 2012 Dec 21. J Cell Sci. 2013. PMID: 23264737 Free PMC article. - Centrosomal localization of cyclin E-Cdk2 is required for initiation of DNA synthesis.
Ferguson RL, Maller JL. Ferguson RL, et al. Curr Biol. 2010 May 11;20(9):856-60. doi: 10.1016/j.cub.2010.03.028. Epub 2010 Apr 22. Curr Biol. 2010. PMID: 20399658 Free PMC article. - RHAMM is a centrosomal protein that interacts with dynein and maintains spindle pole stability.
Maxwell CA, Keats JJ, Crainie M, Sun X, Yen T, Shibuya E, Hendzel M, Chan G, Pilarski LM. Maxwell CA, et al. Mol Biol Cell. 2003 Jun;14(6):2262-76. doi: 10.1091/mbc.e02-07-0377. Epub 2003 Mar 20. Mol Biol Cell. 2003. PMID: 12808028 Free PMC article.
References
- Dasgupta M., Honeycutt, T. and Blumenthal, D.K. (1989) The γ-subunit of skeletal muscle phosphorylase kinase contains two noncontiguous domains that act in concert to bind calmodulin. J. Biol. Chem., 264, 17156–17163. - PubMed
- Diviani D., Langeberg, L.K., Doxsey, S.J. and Scott, J.D. (2000) Pericentrin anchors protein kinase A at the centrosome through a newly identified RII-binding domain. Curr. Biol., 10, 417–420. - PubMed
- Doxsey S.J., Stein, P., Evans, L., Calarco, P.D. and Kirschner, M. (1994) Pericentrin, a highly conserved centrosome protein involved in microtubule organization. Cell, 76, 639–650. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous