Propagation of intercellular calcium waves in retinal astrocytes and Müller cells - PubMed (original) (raw)
Propagation of intercellular calcium waves in retinal astrocytes and Müller cells
E A Newman. J Neurosci. 2001.
Abstract
Intercellular Ca(2+) waves are believed to propagate through networks of glial cells in culture in one of two ways: by diffusion of IP(3) between cells through gap junctions or by release of ATP, which functions as an extracellular messenger. Experiments were conducted to determine the mechanism of Ca(2+) wave propagation between glial cells in an intact CNS tissue. Calcium waves were imaged in the acutely isolated rat retina with the Ca(2+) indicator dye fluo-4. Mechanical stimulation of astrocyte somata evoked Ca(2+) waves that propagated through both astrocytes and Müller cells. Octanol (0.5 mm), which blocks coupling between astrocytes and Müller cells, did not reduce propagation into Müller cells. Purinergic receptor antagonists suramin (100 microm), PPADS (20-50 microm), and apyrase (80 U/ml), in contrast, substantially reduced wave propagation into Müller cells (wave radii reduced to 16-61% of control). Suramin also reduced wave propagation from Müller cell to Müller cell (51% of control). Purinergic antagonists reduced wave propagation through astrocytes to a lesser extent (64-81% of control). Mechanical stimulation evoked the release of ATP, imaged with the luciferin-luciferase bioluminescence assay. Peak ATP concentration at the surface of the retina averaged 78 microm at the stimulation site and 6.8 microm at a distance of 100 microm. ATP release propagated outward from the stimulation site with a velocity of 41 microm/sec, somewhat faster than the 28 microm/sec velocity of Ca(2+) waves. Ejection of 3 microm ATP onto the retinal surface evoked propagated glial Ca(2+) waves. Together, these results indicate that Ca(2+) waves are propagated through retinal glial cells by two mechanisms. Waves are propagated through astrocytes principally by diffusion of an internal messenger, whereas waves are propagated from astrocytes to Müller cells and from Müller cells to other Müller cells primarily by the release of ATP.
Figures
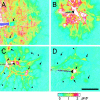
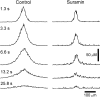
Fig. 1.
Propagation of intercellular Ca2+ waves in retinal glial cells. A, Control. A Ca2+ wave propagates through both astrocytes and Müller cells, with large Ca2+increases occurring in both types of glial cells. [The apparent absence of Ca2+ increases in two astrocytes (arrows) is an artifact; the Ca2+signal in these cells was saturated before stimulation.]B, Octanol, 0.5 m
m
. A Ca2+ wave propagates through both astrocytes and Müller cells. C, Suramin, 100 μ
m
. A Ca2+ wave propagates from the stimulated astrocyte into other astrocyte somata (arrows) and processes (arrowheads), but not into Müller cells (the_blue_ regions between astrocytes).D, Apyrase, 80 U/ml. A Ca2+ wave propagates into several astrocyte somata (arrows) and processes (arrowheads), but not into Müller cells. Waves were evoked by mechanical stimulation of astrocyte somata. The stimulating probe is seen at the left in each panel. Recordings were from eyecups. Scale bar, 50 μm. The pseudocolor ratio images were calculated as described in Materials and Methods. The pseudocolor scale, at the bottom, indicates fluorescence ratio values for this and subsequent figures.
Fig. 2.
Propagation of Ca2+ waves from astrocytes to Müller cells. A and C_show fluorescence intensity (arbitrary units) from selected regions of astrocytes (1, 3) and Müller cells (2, 4). The location of each region is indicated in the fluorescence images in B and_D. A, B, Control. Stimulation of an astrocyte soma evokes a wave that propagates rapidly into adjacent Müller cells. Near the stimulated soma (*) the wave propagates from the astrocyte process (1) into an adjacent Müller cell (2) with a delay of 1.1 sec. Farther from the soma the delay in propagation from the astrocyte process (3) to a Müller cell (4) is 1.3 sec. C,D, PPADS, 50 μ
m
. PPADS impairs astrocyte-to-Müller cell propagation. Near the stimulated soma (*) the wave propagates from the astrocyte process (1) to an adjacent Müller cell (2) with a delay of 2.3 sec. (The secondary rise in Ca2+ in region 1 represents the arrival of the Ca2+ wave in the Müller cells underneath the astrocyte process.) The wave propagates into the soma of a nearby astrocyte (3) but fails to invade an adjacent Müller cell (4). Recordings are from eyecups. In A and C the small dots mark the onset of Ca2+ increases, and_vertical arrows_ indicate the time of mechanical stimulation. Scale bar in D, 50 μm.
Fig. 3.
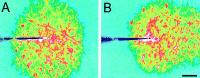
Calcium wave propagation is altered by superfusate flow. A, Superfusate flow turned off. Propagation is symmetric. B, Superfusate flow from _left_to right. Propagation is highly asymmetric. Propagation in the direction of superfusate flow is greatly extended, whereas propagation in the direction opposite the flow is reduced. The two images were obtained from nearby regions of the same retina. Waves were evoked by mechanical stimulation. The tip of the stimulating probe is near the center of the images. Shown are recordings from a whole-mount retina. Scale bar, 50 μm.
Fig. 4.
ATP receptor antagonist blocks asymmetric wave propagation. Superfusate flow is from top left to bottom right in all three trials.A, Control. Superfusate flow causes asymmetric wave propagation. B, Suramin, 100 μ
m
. The purinergic receptor blocker eliminates the asymmetric wave propagation despite the continued superfusate flow. C, Recovery. After washout of suramin (39 min) the asymmetry in wave propagation returns. Waves were evoked by mechanical stimulation. The three images were obtained from nearby regions of the same retina. Shown are recordings from a whole-mount retina. Scale bar, 50 μm.
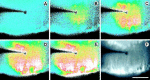
Fig. 5.
Propagation of an intercellular Ca2+ wave in Müller cells. Shown are images from a retinal slice, viewed looking down onto the cut surface of the slice. A–E, Pseudocolor images of Ca2+ wave propagation through Müller cells evoked by mechanical stimulation. The wave propagates in all directions within Müller cells, invading cell somata and endfeet, where large Ca2+ increases are seen. Elapsed time after stimulation in A–E: 0, 1.3, 2.0, 3.0, and 5.0 sec.F, A fluorescence image of the slice showing labeled Müller cells. Müller cell somata in the inner nuclear layer are at the top of the image. Müller cell endfeet at the vitreal surface of the retina are at the bottom. Müller cell processes (thin vertical lines) within the inner plexiform layer were stimulated by the probe. Scale bar, 50 μm.
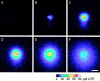
Fig. 6.
Propagation of a wave of ATP release from the retina. ATP release was monitored via the luciferin–luciferase bioluminescence assay. ATP concentration at the retinal surface is indicated by the pseudocolor scale at the_bottom_. The ATP release wave was evoked by a mechanical stimulus identical to that used to elicit Ca2+waves. Elapsed time after stimulation in A–F: 0, 0.7, 2.0, 4.0, 7.9, and 16.5 sec. Shown are images from a whole-mount retina. Scale bar, 100 μm.
Fig. 7.
ATP receptor antagonist blocks propagation of ATP release wave. Spatial profiles of ATP concentration at the retinal surface are shown for five time points after stimulation.Left, Control trial. Immediately after stimulation (1.3 sec) ATP release is confined to a region near the stimulation site (center of trace). At later times ATP release occurs at greater distances from the stimulation site. Right, Suramin, 100 μ
m
. ATP release is confined to a small region near the stimulation site, even at later times. ATP release does not propagate to neighboring regions. Shown are recordings from whole-mount retinas.
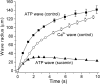
Fig. 8.
Comparison of ATP release wave and Ca2+ wave propagation. Wave radius is plotted as a function of time after stimulation for control ATP release waves (▪), ATP release waves in the presence of 100 μ
m
suramin (▴), and control Ca2+ waves (○). Propagation of control ATP release waves precedes Ca2+ waves by ∼25 μm and ∼0.9 sec during the first seconds after stimulation. In the presence of suramin the ATP release fails to spread beyond 30 μm from the stimulation site. Threshold for detecting the leading edge of ATP waves was 3 μ
m
ATP. Threshold for Ca2+ waves was a Δ_F_/F increase of 0.6. Means ± SEM are shown. n = 8, 6, and 8 for control ATP, suramin ATP, and Ca2+ waves, respectively. Shown are recordings from whole-mount retinas.
Similar articles
- Calcium increases in retinal glial cells evoked by light-induced neuronal activity.
Newman EA. Newman EA. J Neurosci. 2005 Jun 8;25(23):5502-10. doi: 10.1523/JNEUROSCI.1354-05.2005. J Neurosci. 2005. PMID: 15944378 Free PMC article. - Two different mechanosensitive calcium responses in Müller glial cells of the guinea pig retina: Differential dependence on purinergic receptor signaling.
Agte S, Pannicke T, Ulbricht E, Reichenbach A, Bringmann A. Agte S, et al. Glia. 2017 Jan;65(1):62-74. doi: 10.1002/glia.23054. Epub 2016 Oct 5. Glia. 2017. PMID: 27706854 - Müller cell Ca2+ waves evoked by purinergic receptor agonists in slices of rat retina.
Li Y, Holtzclaw LA, Russell JT. Li Y, et al. J Neurophysiol. 2001 Feb;85(2):986-94. doi: 10.1152/jn.2001.85.2.986. J Neurophysiol. 2001. PMID: 11160528 - Role of Purines in Müller Glia.
Reichenbach A, Bringmann A. Reichenbach A, et al. J Ocul Pharmacol Ther. 2016 Oct;32(8):518-533. doi: 10.1089/jop.2016.0131. J Ocul Pharmacol Ther. 2016. PMID: 27754822 Review. - Purinergic signaling involved in Müller cell function in the mammalian retina.
Wurm A, Pannicke T, Iandiev I, Francke M, Hollborn M, Wiedemann P, Reichenbach A, Osborne NN, Bringmann A. Wurm A, et al. Prog Retin Eye Res. 2011 Sep;30(5):324-42. doi: 10.1016/j.preteyeres.2011.06.001. Epub 2011 Jun 14. Prog Retin Eye Res. 2011. PMID: 21689780 Review.
Cited by
- Protective effect of insulin and glucose at different concentrations on penicillin-induced astrocyte death on the primer astroglial cell line.
Özdemir MB, Akça H, Erdoğan Ç, Tokgün O, Demiray A, Semin F, Becerir C. Özdemir MB, et al. Neural Regen Res. 2012 Aug 25;7(24):1895-9. doi: 10.3969/j.issn.1673-5374.2012.24.008. Neural Regen Res. 2012. PMID: 25624816 Free PMC article. - Energy metabolism of the visual system.
Wong-Riley MT. Wong-Riley MT. Eye Brain. 2010;2:99-116. doi: 10.2147/EB.S9078. Epub 2010 Jul 22. Eye Brain. 2010. PMID: 23226947 Free PMC article. - Neurons respond directly to mechanical deformation with pannexin-mediated ATP release and autostimulation of P2X7 receptors.
Xia J, Lim JC, Lu W, Beckel JM, Macarak EJ, Laties AM, Mitchell CH. Xia J, et al. J Physiol. 2012 May 15;590(10):2285-304. doi: 10.1113/jphysiol.2012.227983. Epub 2012 Mar 12. J Physiol. 2012. PMID: 22411013 Free PMC article. - Subcellular propagation of calcium waves in Müller glia does not require autocrine/paracrine purinergic signaling.
Phuong TT, Yarishkin O, Križaj D. Phuong TT, et al. Channels (Austin). 2016 Sep 2;10(5):421-427. doi: 10.1080/19336950.2016.1193276. Epub 2016 May 24. Channels (Austin). 2016. PMID: 27221769 Free PMC article. - Simulation of calcium signaling in fine astrocytic processes: Effect of spatial properties on spontaneous activity.
Denizot A, Arizono M, Nägerl UV, Soula H, Berry H. Denizot A, et al. PLoS Comput Biol. 2019 Aug 19;15(8):e1006795. doi: 10.1371/journal.pcbi.1006795. eCollection 2019 Aug. PLoS Comput Biol. 2019. PMID: 31425510 Free PMC article.
References
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Glutamate-dependent astrocyte modulation of synaptic transmission between cultured hippocampal neurons. Eur J Neurosci. 1998a;10:2129–2142. - PubMed
- Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Lodi Rizzini B, Pozzan T, Volterra A. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature. 1998;391:281–285. - PubMed
- Boitano S, Dirksen ER. Intercellular propagation of calcium waves mediated by inositol trisphosphate. Science. 1992;258:292–295. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous