Limbic-cortical-ventral striatal activation during retrieval of a discrete cocaine-associated stimulus: a cellular imaging study with gamma protein kinase C expression - PubMed (original) (raw)
Limbic-cortical-ventral striatal activation during retrieval of a discrete cocaine-associated stimulus: a cellular imaging study with gamma protein kinase C expression
K L Thomas et al. J Neurosci. 2001.
Abstract
We investigated the neuronal activation associated with reexposure to a discrete cocaine-associated stimulus using in situ hybridization to quantify the expression of the plasticity-regulated gene, gamma protein kinase C (gamma PKC), in the limbic-cortical-ventral striatal system. Groups of rats were trained to self-administer cocaine paired with a light stimulus (Paired) or paired with an auditory stimulus but also receiving light presentations yoked to those in the Paired group (Unpaired). Additional groups received noncontingent cocaine-light pairings (Pavlovian) or saline-light pairings (Saline) that were yoked to the Paired group. After acquisition of self-administration by the Paired and Unpaired groups, all groups had a 3 d drug- and training-free period before being reexposed to noncontingent presentations of the light conditioning stimulus during a 5 min test session in the training context. There were four major patterns of results for regional gamma PKC expression 2 hr later. (1) Changes occurred only in groups in which the light was predictive of cocaine. (2) Increases were seen in the amygdala, but decreases were seen in the medial prefrontal cortex. (3) No changes were seen in the hippocampus. (4) Although changes were observed in the basal and central nuclei of the amygdala and the prelimbic cortex in both the Paired and Pavlovian groups, additional changes were observed in the nucleus accumbens core, lateral amygdala, and anterior cingulate cortex in the Pavlovian group. These results suggest not only that regionally selective alterations in gamma PKC expression are an index of the retrieval of Pavlovian associations formed between a drug and a discrete stimulus, but also that a distinct neural circuitry may underlie Pavlovian stimulus-reward associations in cocaine-experienced rats.
Figures
Fig. 1.
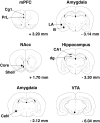
Schematic representation of the brain regions targeted for grain density measurement. Bilateral measurements in the mPFC (Cg1 and PrL), NAcc (Core and Shell), amygdala (CeN, LA, and B), hippocampus (dg and CA1), and VTA were made as indicated. Coronal sections are marked “+” for anterior and “−” for posterior to bregma (Paxinos and Watson, 1997).
Fig. 2.
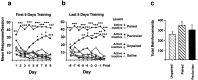
Acquisition of intravenous cocaine SA.a, b, The mean number of responses on the active (cocaine or saline SA,) or inactive (no programmed consequence) levers in the 2 hr training sessions in the first 9 d of training (a) or the last 9 d of training (b). c, The total number of cocaine (0.25 mg base/0.1 ml saline) infusions in rats self-administering (Unpaired and _Paired_groups) or receiving yoked administrations (Pavlovian) of the drug. *p < 0.05, **p < 0.01, ***p < 0.001, in comparison with the responses at the inactive lever; Sidak's test. Error bars indicate SEM.
Fig. 3.
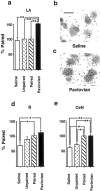
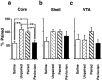
CS-induced γ PKC expression in the amygdala.a, The percentage change in the density of silver grains 2 hr after light stimulus presentation in neurons of the lateral amygdala in rats trained with noncontingent light CS–cocaine (Pavlovian group) or light CS–saline (Saline group) pairings and in rats trained to self-administer cocaine associated with a tone CS (Unpaired group) relative to the density measured in rats trained to self-administer cocaine paired with the light CS (Paired group). b, c, Representative photomicrographs showing the individual silver grains over thionine-counterstained LA amygdala neurons in a section from rats from Saline (b) and Pavlovian (c) groups. Scale bar, 15 μm. d,e, The percentage change in the density of silver grains 2 hr after light stimulus presentation in neurons of the basal (d) and CeN (e) subregions of the amygdala. *p < 0.05, **p < 0.01; Tukey's test. Error bars indicate SEM.
Fig. 4.
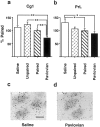
CS-induced γ PKC expression in the medial prefrontal cortex. a, b, The neuronal density of silver grains in Cg1 (a) or PrL (b) regions measured 2 hr after presentation of a light stimulus to rats trained to self-administer cocaine and paired either with a light CS (Paired group) or tone CS (Unpaired group), or to rats that had received noncontingent cocaine and light CS (Pavlovian group) or saline and light CS (Saline group) administrations during training. The results are shown relative to the density measured in the Paired group (100%). *p < 0.05, **p < 0.01; Tukey's test. Error bars indicate SEM. c, d, Representative photomicrographs showing the individual silver grains over thionine-counterstained PrL neurons in a section from rats from Saline (c) and Pavlovian (d) groups. Scale bar, 15 μm.
Fig. 5.
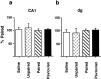
CS-induced γ PKC expression in the hippocampus.a, b, The expression of γ PKC in CA1 (a) and dg (b) neurons in rats trained to self-administer cocaine paired with a light CS (Paired group) or a tone CS (_Unpaired_group) or in rats trained with noncontingent administration of cocaine and light CS (Pavlovian group) or saline and light CS (Saline group). The results are presented as silver grain density relative to the Paired group (100%). Error bars indicate SEM.
Fig. 6.
CS-induced γ PKC expression in mesoaccumbal DA system. The density of silver grains, standardized to the number of grains per neuron, measured in animals self-administering cocaine and receiving paired light CS (Paired group) in the core of the NAcc (a), the shell of the NAcc (b), and VTA 2 hr after presentation of a light stimulus (c) in groups of rats that, during training, received either yoked noncontingent presentations of the light CS and cocaine (Pavlovian group) or saline (Saline group), or rats that had self-administered cocaine paired with an auditory tone (Unpaired group). *p < 0.05, **p < 0.01; Tukey's test. Error bars indicate SEM.
Similar articles
- Induction of the learning and plasticity-associated gene Zif268 following exposure to a discrete cocaine-associated stimulus.
Thomas KL, Arroyo M, Everitt BJ. Thomas KL, et al. Eur J Neurosci. 2003 May;17(9):1964-72. doi: 10.1046/j.1460-9568.2003.02617.x. Eur J Neurosci. 2003. PMID: 12752796 - Context-induced relapse to cocaine seeking after punishment-imposed abstinence is associated with activation of cortical and subcortical brain regions.
Pelloux Y, Hoots JK, Cifani C, Adhikary S, Martin J, Minier-Toribio A, Bossert JM, Shaham Y. Pelloux Y, et al. Addict Biol. 2018 Mar;23(2):699-712. doi: 10.1111/adb.12527. Epub 2017 Jun 29. Addict Biol. 2018. PMID: 28661034 Free PMC article. - Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment.
Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. Neisewander JL, et al. J Neurosci. 2000 Jan 15;20(2):798-805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. J Neurosci. 2000. PMID: 10632609 Free PMC article. - Changes in endocannabinoid and N-acylethanolamine levels in rat brain structures following cocaine self-administration and extinction training.
Bystrowska B, Smaga I, Frankowska M, Filip M. Bystrowska B, et al. Prog Neuropsychopharmacol Biol Psychiatry. 2014 Apr 3;50:1-10. doi: 10.1016/j.pnpbp.2013.12.002. Epub 2013 Dec 12. Prog Neuropsychopharmacol Biol Psychiatry. 2014. PMID: 24334211 - Neural substrates of cocaine-cue associations that trigger relapse.
See RE. See RE. Eur J Pharmacol. 2005 Dec 5;526(1-3):140-6. doi: 10.1016/j.ejphar.2005.09.034. Epub 2005 Oct 25. Eur J Pharmacol. 2005. PMID: 16253228 Review.
Cited by
- Group I metabotropic glutamate receptor-mediated activation of PKC gamma in the nucleus accumbens core promotes the reinstatement of cocaine seeking.
Schmidt HD, Kimmey BA, Arreola AC, Pierce RC. Schmidt HD, et al. Addict Biol. 2015 Mar;20(2):285-96. doi: 10.1111/adb.12122. Epub 2014 Feb 9. Addict Biol. 2015. PMID: 24506432 Free PMC article. - Cocaine-seeking is associated with PKC-dependent reduction of excitatory signaling in accumbens shell D2 dopamine receptor-expressing neurons.
Ortinski PI, Briand LA, Pierce RC, Schmidt HD. Ortinski PI, et al. Neuropharmacology. 2015 May;92:80-9. doi: 10.1016/j.neuropharm.2015.01.002. Epub 2015 Jan 14. Neuropharmacology. 2015. PMID: 25596492 Free PMC article. - Cocaine-induced neuroadaptations in glutamate transmission: potential therapeutic targets for craving and addiction.
Schmidt HD, Pierce RC. Schmidt HD, et al. Ann N Y Acad Sci. 2010 Feb;1187:35-75. doi: 10.1111/j.1749-6632.2009.05144.x. Ann N Y Acad Sci. 2010. PMID: 20201846 Free PMC article. Review. - The structural basis for mapping behavior onto the ventral striatum and its subdivisions.
Meredith GE, Baldo BA, Andrezjewski ME, Kelley AE. Meredith GE, et al. Brain Struct Funct. 2008 Sep;213(1-2):17-27. doi: 10.1007/s00429-008-0175-3. Epub 2008 Feb 7. Brain Struct Funct. 2008. PMID: 18256852 Free PMC article. Review. - Repeated amphetamine administration induces Fos in prefrontal cortical neurons that project to the lateral hypothalamus but not the nucleus accumbens or basolateral amygdala.
Morshedi MM, Meredith GE. Morshedi MM, et al. Psychopharmacology (Berl). 2008 Apr;197(2):179-89. doi: 10.1007/s00213-007-1021-7. Epub 2007 Dec 14. Psychopharmacology (Berl). 2008. PMID: 18080115 Free PMC article.
References
- Abeliovich A, Chen C, Goga Y, Silva AJ, Stevens CF, Tonegawa S. Modified hippocampal long-term potentiation in PKC γ mutant mice. Cell. 1993a;75:1253–1262. - PubMed
- Abeliovich A, Paylor R, Chen C, Kim JJ, Wehner JM, Tonegawa S. PKC γ mutant mice exhibit mild deficits in spatial and contextual learning. Cell. 1993b;75:1263–1271. - PubMed
- Abraham WC, Mason SE, Demmer J, Williams JM, Richardson CL, Tate WP, Lawlor P, Dragunow M. Correlations between immediate early gene induction and the persistence of long-term potentiation. Neuroscience. 1993;56:717–727. - PubMed
- Arroyo M, Markou A, Robbins TW, Everitt BJ. Acquisition, maintenance and reinstatement of intravenous cocaine self-administration under a second-order schedule of reinforcement in rats: effects of conditioned cues and continuous access to cocaine. Psychopharmacology (Berl) 1998;140:331–344. - PubMed
- Baldwin AE, Holahan MR, Sadeghian K, Kelley AE. N-Methyl-d-aspartate-dependent plasticity within a distributed corticostriatal network mediates appetitve instrumental learning. Behav Neurosci. 2000;114:84–98. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources