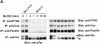Convergence of alpha(v)beta(3) integrin- and macrophage colony stimulating factor-mediated signals on phospholipase Cgamma in prefusion osteoclasts - PubMed (original) (raw)
Convergence of alpha(v)beta(3) integrin- and macrophage colony stimulating factor-mediated signals on phospholipase Cgamma in prefusion osteoclasts
I Nakamura et al. J Cell Biol. 2001.
Abstract
The macrophage colony stimulating factor (M-CSF) and alpha(v)beta(3) integrins play critical roles in osteoclast function. This study examines M-CSF- and adhesion-induced signaling in prefusion osteoclasts (pOCs) derived from Src-deficient and wild-type mice. Src-deficient cells attach to but do not spread on vitronectin (Vn)-coated surfaces and, contrary to wild-type cells, their adhesion does not lead to tyrosine phosphorylation of molecules activated by adhesion, including PYK2, p130(Cas), paxillin, and PLC-gamma. However, in response to M-CSF, Src(-/-) pOCs spread and migrate on Vn in an alpha(v)beta(3)-dependent manner. Involvement of PLC-gamma activation is suggested by using a PLC inhibitor, U73122, which blocks both adhesion- and M-CSF-mediated cell spreading. Furthermore, in Src(-/-) pOCs M-CSF, together with filamentous actin, causes recruitment of beta(3) integrin and PLC-gamma to adhesion contacts and induces stable association of beta(3) integrin with PLC-gamma, phosphatidylinositol 3-kinase, and PYK2. Moreover, direct interaction of PYK2 and PLC-gamma can be induced by either adhesion or M-CSF, suggesting that this interaction may enable the formation of integrin-associated complexes. Furthermore, this study suggests that in pOCs PLC-gamma is a common downstream mediator for adhesion and growth factor signals. M-CSF-initiated signaling modulates the alpha(v)beta(3) integrin-mediated cytoskeletal reorganization in prefusion osteoclasts in the absence of c-Src, possibly via PLC-gamma.
Figures
Figure 1
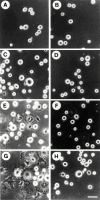
Src−/− pOCs do not spread on Vn-coated dishes. Src+/? (A, C, E, and G) and Src−/− (B, D, F, and H) pOCs were plated on Vn (20 μg/ml). After culture for 5 (A and B), 15 (C and D), 30 (E and F), and 60 (G and H) min, cells were fixed and photographed. (I) To quantify cell area, the periphery of each cell was outlined and the total planar area was calculated, using an image analysis system (Empire Imaging Systems). Data are expressed as the means of ± SEM of >50 cells.
Figure 1
Src−/− pOCs do not spread on Vn-coated dishes. Src+/? (A, C, E, and G) and Src−/− (B, D, F, and H) pOCs were plated on Vn (20 μg/ml). After culture for 5 (A and B), 15 (C and D), 30 (E and F), and 60 (G and H) min, cells were fixed and photographed. (I) To quantify cell area, the periphery of each cell was outlined and the total planar area was calculated, using an image analysis system (Empire Imaging Systems). Data are expressed as the means of ± SEM of >50 cells.
Figure 2
M-CSF induces cell spreading of Src−/− pOCs on Vn-coated dishes. (A) Src−/− pOCs were plated on Vn-coated dishes in the absence of serum for 60 min, cells were then treated with 5 nM M-CSF for 0 (a), 2 (b), 5 (c), 15 (d), and 30 (e) min, without or with (f) 100 nM wortmannin. (B) Src+/? (a) and Src−/− (b and c) pOCs were plated on Vn for 60 min, untreated (a and b) or treated with 5 nM M-CSF treatment for additional 30 min (c). Cells were fixed and stained with rhodamine-conjugated phalloidin. Bars: (A) 10 μm; (B) 5 μm.
Figure 3
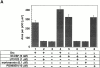
M-CSF–induced cell spreading of Src−/− pOCs is dependent on αvβ3 integrin. Src−/− pOCs were plated on Vn or PL in serum-free condition. After 60 min, cells were treated with 5 nM M-CSF for 30 min in the absence or presence of echistatin (1 nM). Cells were fixed and stained for TRAP activity, followed by quantitating cell area as described above. Data are presented as means ± SEM and n = 50 cells per group.
Figure 6
Adhesion-induced tyrosine phosphorylation of PYK2, Cas, paxillin, and PLC-γ in Src+/? and Src−/− prefusion osteoclast-like cells. (A) Src+/? pOCs were kept in suspension for 60 min or plated on Vn-coated dishes for the indicated periods in the absence of serum. Total cell lysates were immunoprecipitated (IP) with anti-PYK2, anti-Cas, anti–paxillin, anti–PLC-γ1 and 2, and anti-Src antibodies, followed by immunoblotting with anti–phosphotyrosine (pTyr) antibody (left). The same membranes were reblotted with anti-PYK2, anti-Cas, anti–paxillin, anti–PLC-γ1 and 2, and anti-Src antibodies (right). (B) Src+/? or Src−/− pOCs were kept in cell suspension or plated on Vn for 60 min. Total cell lysates were subjected to immunoprecipitation as described above. S, suspension; A, attached. (C) Src+/? or Src−/− pOCs (1.0 × 106 cells) were either plated on Vn-coated dishes for 60 min (lanes 1 and 3) or re-cultured with equal number of vitamin D3-treated MB1.8 cells on tissue culture dishes to generate OCLs (lanes 2 and 4). After 12 h, OCLs were purified as described in Materials and Methods. Cell lysates were immunoprecipitated with anti-paxillin, followed by blotting with p-Tyr and anti-paxillin. Arrowhead shows the position of paxillin.
Figure 6
Adhesion-induced tyrosine phosphorylation of PYK2, Cas, paxillin, and PLC-γ in Src+/? and Src−/− prefusion osteoclast-like cells. (A) Src+/? pOCs were kept in suspension for 60 min or plated on Vn-coated dishes for the indicated periods in the absence of serum. Total cell lysates were immunoprecipitated (IP) with anti-PYK2, anti-Cas, anti–paxillin, anti–PLC-γ1 and 2, and anti-Src antibodies, followed by immunoblotting with anti–phosphotyrosine (pTyr) antibody (left). The same membranes were reblotted with anti-PYK2, anti-Cas, anti–paxillin, anti–PLC-γ1 and 2, and anti-Src antibodies (right). (B) Src+/? or Src−/− pOCs were kept in cell suspension or plated on Vn for 60 min. Total cell lysates were subjected to immunoprecipitation as described above. S, suspension; A, attached. (C) Src+/? or Src−/− pOCs (1.0 × 106 cells) were either plated on Vn-coated dishes for 60 min (lanes 1 and 3) or re-cultured with equal number of vitamin D3-treated MB1.8 cells on tissue culture dishes to generate OCLs (lanes 2 and 4). After 12 h, OCLs were purified as described in Materials and Methods. Cell lysates were immunoprecipitated with anti-paxillin, followed by blotting with p-Tyr and anti-paxillin. Arrowhead shows the position of paxillin.
Figure 6
Adhesion-induced tyrosine phosphorylation of PYK2, Cas, paxillin, and PLC-γ in Src+/? and Src−/− prefusion osteoclast-like cells. (A) Src+/? pOCs were kept in suspension for 60 min or plated on Vn-coated dishes for the indicated periods in the absence of serum. Total cell lysates were immunoprecipitated (IP) with anti-PYK2, anti-Cas, anti–paxillin, anti–PLC-γ1 and 2, and anti-Src antibodies, followed by immunoblotting with anti–phosphotyrosine (pTyr) antibody (left). The same membranes were reblotted with anti-PYK2, anti-Cas, anti–paxillin, anti–PLC-γ1 and 2, and anti-Src antibodies (right). (B) Src+/? or Src−/− pOCs were kept in cell suspension or plated on Vn for 60 min. Total cell lysates were subjected to immunoprecipitation as described above. S, suspension; A, attached. (C) Src+/? or Src−/− pOCs (1.0 × 106 cells) were either plated on Vn-coated dishes for 60 min (lanes 1 and 3) or re-cultured with equal number of vitamin D3-treated MB1.8 cells on tissue culture dishes to generate OCLs (lanes 2 and 4). After 12 h, OCLs were purified as described in Materials and Methods. Cell lysates were immunoprecipitated with anti-paxillin, followed by blotting with p-Tyr and anti-paxillin. Arrowhead shows the position of paxillin.
Figure 4
M-CSF induces cell migration of Src−/− pOCs as well as wild-type cells. The motility of Src+/? and Src−/− pOCs was monitored using time-lapse video microscopy, as described in Materials and Methods. M-CSF (1 nM) was supplied through a micropipette to induce chemotaxis. Cells were observed using an inverted phase–contrast microscope coupled to a video camera and time-lapse video recorder. Images of cells were digitized using a computer-based image analysis system. Migration activity (net translocation of the cell center over a period of 4 h) was quantified (filled circles). 15 out of 26 (58%) Src+/? cells and 10 out of 19 (53%) Src−/− cells migrated towards the source of M-CSF. Means ± SEM (open circles) of migrating distance of wild-type and Src−/− cells are shown.
Figure 7
M-CSF–induced intracellular signaling in Src−/− prefusion osteoclast-like cells. Src+/? and Src−/− pOCs were kept in suspension or plated on Vn for 60 min in the absence of serum, followed by treatment with 5 nM M-CSF for the indicated periods with or without PI 3-kinase inhibitors. (A) Total cell lysates were immunoprecipitated with anti-PYK2, anti-Cas, antipaxillin, and anti-Src, and blotted with antiphosphotyrosine (pTyr, left), anti-PYK2, anti-Cas, antipaxillin, and anti-Src antibodies (right). (B) Lysates were immunoprecipitated with anti–PLC-γ2, blotted with anti-pTyr (top), then with anti–PLC-γ2 antibodies (middle). Part of the total cell lysates were used for immunodetection of c-Src (bottom). (C) Lysates were immunoprecipitated with anti-Akt/PKB, blotted with anti–phospho-Akt/PKB, or anti-Akt/PKB antibodies. S, suspension; A, attached.
Figure 7
M-CSF–induced intracellular signaling in Src−/− prefusion osteoclast-like cells. Src+/? and Src−/− pOCs were kept in suspension or plated on Vn for 60 min in the absence of serum, followed by treatment with 5 nM M-CSF for the indicated periods with or without PI 3-kinase inhibitors. (A) Total cell lysates were immunoprecipitated with anti-PYK2, anti-Cas, antipaxillin, and anti-Src, and blotted with antiphosphotyrosine (pTyr, left), anti-PYK2, anti-Cas, antipaxillin, and anti-Src antibodies (right). (B) Lysates were immunoprecipitated with anti–PLC-γ2, blotted with anti-pTyr (top), then with anti–PLC-γ2 antibodies (middle). Part of the total cell lysates were used for immunodetection of c-Src (bottom). (C) Lysates were immunoprecipitated with anti-Akt/PKB, blotted with anti–phospho-Akt/PKB, or anti-Akt/PKB antibodies. S, suspension; A, attached.
Figure 7
M-CSF–induced intracellular signaling in Src−/− prefusion osteoclast-like cells. Src+/? and Src−/− pOCs were kept in suspension or plated on Vn for 60 min in the absence of serum, followed by treatment with 5 nM M-CSF for the indicated periods with or without PI 3-kinase inhibitors. (A) Total cell lysates were immunoprecipitated with anti-PYK2, anti-Cas, antipaxillin, and anti-Src, and blotted with antiphosphotyrosine (pTyr, left), anti-PYK2, anti-Cas, antipaxillin, and anti-Src antibodies (right). (B) Lysates were immunoprecipitated with anti–PLC-γ2, blotted with anti-pTyr (top), then with anti–PLC-γ2 antibodies (middle). Part of the total cell lysates were used for immunodetection of c-Src (bottom). (C) Lysates were immunoprecipitated with anti-Akt/PKB, blotted with anti–phospho-Akt/PKB, or anti-Akt/PKB antibodies. S, suspension; A, attached.
Figure 5
PLC and PI 3-kinase inhibitors block adhesion-induced and M-CSF–induced cell spreading of prefusion osteoclast-like cells. (A) Src+/? pOCs (1, 2, and 4) and Src−/− pOCs (3 and 5–8) were plated on Vn under serum-free condition. After 60 min, cells were treated with (4–8) or without (1–3) M-CSF for 30 min. U73122 (2 and 6), wortmannin (7), or PD98059 (8) was preincubated for 40 min before M-CSF was added. Cells were fixed, stained for TRAP, and the total planar area was calculated as described in Materials and Methods. Data are presented as means ± SEM; n = 50 cells per group. (B) Src+/? and Src−/− pOCs were kept in suspension or plated on Vn for 60 min in the absence of serum, followed by the treatment with 5 nM M-CSF for indicated periods with or without 10 μM PD98059. Lysates were blotted with anti–phospho-ERK (top), followed by anti-ERK and anti-Src (bottom). Susp., suspension; Att., attachment.
Figure 5
PLC and PI 3-kinase inhibitors block adhesion-induced and M-CSF–induced cell spreading of prefusion osteoclast-like cells. (A) Src+/? pOCs (1, 2, and 4) and Src−/− pOCs (3 and 5–8) were plated on Vn under serum-free condition. After 60 min, cells were treated with (4–8) or without (1–3) M-CSF for 30 min. U73122 (2 and 6), wortmannin (7), or PD98059 (8) was preincubated for 40 min before M-CSF was added. Cells were fixed, stained for TRAP, and the total planar area was calculated as described in Materials and Methods. Data are presented as means ± SEM; n = 50 cells per group. (B) Src+/? and Src−/− pOCs were kept in suspension or plated on Vn for 60 min in the absence of serum, followed by the treatment with 5 nM M-CSF for indicated periods with or without 10 μM PD98059. Lysates were blotted with anti–phospho-ERK (top), followed by anti-ERK and anti-Src (bottom). Susp., suspension; Att., attachment.
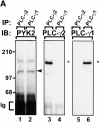
Figure 8
M-CSF–induced association of αvβ3 integrin with signaling molecules in Src−/− prefusion osteoclast-like cells. (A) Cell adhesion and M-CSF induce the association of β3 integrins with signaling molecules in pOCs. Src+/? and Src−/− pOCs (1.5 × 106 cells per condition) were plated on PL- or Vn-coated dishes. After culture for 60 min, Src−/− cells were treated with or without 5 nM M-CSF for 5 min. Total cell lysates were immunoprecipitated (IP) with anti–β3 integrin antibodies, followed by immunoblotting (IB) with anti–PLC-γ2 (lanes 1–4), anti-PYK2 (lanes 5–8), anti–PI 3-kinase (lanes 9–12), anti–c-Src (lanes 13–16), and anti–β3 integrin (lanes 17–20). The molecular masses of marker proteins (in kD) are on the left. Positions of c-Src (arrowhead) and of p85 subunit of PI 3-kinase (asterisk) are as indicated. (B) PLC and PI 3-kinase inhibitors block M-CSF–induced association of αvβ3 integrin with signaling molecules in Src-deficient pOCs. Src−/− pOCs (1.5 × 106 cells per condition) were plated on Vn as described above, followed by incubation with either U73122 (1 μM) or LY294002 (50 μM) for 40 min, then with 5 nM M-CSF. Lysates were immunoprecipitated with hamster anti–murine β3 integrin antibodies (lanes 2–4, 6–8, 10–12, and 14–16) or control hamster IgG (lanes 1, 5, 9, and 13), followed by blotting with anti–PLC-γ2 (lanes 1–4), anti-PYK2 (lanes 5–8), anti–PI 3-kinase (lanes 9–12), or anti–β3 integrin (lanes 13–16). p85 subunit of PI 3-kinase (small arrowheads). C, control hamster IgGs.
Figure 8
M-CSF–induced association of αvβ3 integrin with signaling molecules in Src−/− prefusion osteoclast-like cells. (A) Cell adhesion and M-CSF induce the association of β3 integrins with signaling molecules in pOCs. Src+/? and Src−/− pOCs (1.5 × 106 cells per condition) were plated on PL- or Vn-coated dishes. After culture for 60 min, Src−/− cells were treated with or without 5 nM M-CSF for 5 min. Total cell lysates were immunoprecipitated (IP) with anti–β3 integrin antibodies, followed by immunoblotting (IB) with anti–PLC-γ2 (lanes 1–4), anti-PYK2 (lanes 5–8), anti–PI 3-kinase (lanes 9–12), anti–c-Src (lanes 13–16), and anti–β3 integrin (lanes 17–20). The molecular masses of marker proteins (in kD) are on the left. Positions of c-Src (arrowhead) and of p85 subunit of PI 3-kinase (asterisk) are as indicated. (B) PLC and PI 3-kinase inhibitors block M-CSF–induced association of αvβ3 integrin with signaling molecules in Src-deficient pOCs. Src−/− pOCs (1.5 × 106 cells per condition) were plated on Vn as described above, followed by incubation with either U73122 (1 μM) or LY294002 (50 μM) for 40 min, then with 5 nM M-CSF. Lysates were immunoprecipitated with hamster anti–murine β3 integrin antibodies (lanes 2–4, 6–8, 10–12, and 14–16) or control hamster IgG (lanes 1, 5, 9, and 13), followed by blotting with anti–PLC-γ2 (lanes 1–4), anti-PYK2 (lanes 5–8), anti–PI 3-kinase (lanes 9–12), or anti–β3 integrin (lanes 13–16). p85 subunit of PI 3-kinase (small arrowheads). C, control hamster IgGs.
Figure 9
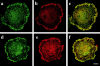
Colocalization of αvβ3 integrin and PLC-γ in M-CSF treated Src−/− prefusion osteoclasts. Src−/− pOCs were plated on Vn-coated glass coverslips for 1 hr, then treated with 5 nM M-CSF for 30 min. Cells were fixed double stained with polyclonal anti-β3 integrin and monoclonal anti-PLC-γ1 or PLC-γ2 antibodies. Pseudocolored confocal microscopic images of β3 integrins (a, d, in green) and double staining of PLC-γ1 (b, in red) or PLC-γ2 (e, in red). Colocalization (c, f, in yellow) appears to be more prominent in the adhesion contacts organized at the spreading edge of the cells. Images merged from optical sections of 4.7 μm thickness at the adhesion surface of the cells. Bar, 10 μm.
Figure 10
M-CSF–dependent association of PLC-γ2 and PYK2 in osteoclasts. Src+/? pOCs were cultured on Vn-coated dishes for 60 min in the absence of serum. (A) Total cell lysates were immunoprecipitated (IP) with anti–PLC-γ2 (lane 1) and anti–PLC-γ1 (lane 2), followed by blotting with anti-PYK2 (left), anti–PLC-γ2 (middle), or anti–PLC-γ1 (right) antibodies. (B) Lysates were immunoprecipitated with anti-PYK2 mAb 11 (lane 1) and anti-PYK2 N-19 antibodies (lane 2), followed by blotting with anti–PLC-γ2 (left) or anti-PYK2 (right). (C) Src+/? pOCs were cultured on PL or Vn for 60 min with or without 1 μM U73122. Lysates were immunoprecipitated with anti–PLC-γ2 and blotted with anti-PYK2 (left), and anti–PLC-γ2 (right) antibodies. (D) Lysates of Src+/? OCLs were incubated with GST fusion proteins containing NH2- and COOH-terminal SH2 domains or SH3 domains of PLC-γ1 and blotted with anti-PYK2 antibodies (top) or incubated with GST fusion proteins of NH2- or COOH-terminal domains or kinase (K) domain of PYK2 and blotted with anti–PLC-γ2 antibodies (bottom). (E) Src−/− pOCs (1.5 × 106 cells per condition) were plated on Vn and treated without and with M-CSF. Adhesion of Src+/? pOCs on Vn was used as control. Lysates were immunoprecipitated with anti–PLC-γ2 and blotted with anti-PYK2, followed by anti–PLC-γ2 antibodies (left) or immunoprecipitated with anti-N terminal PYK2 and blotted with anti–PLC-γ2, followed by anti-PYK2 antibodies (right). Molecular weight markers (in kD) are as indicated on the left. Positions of PYK2 (arrowhead) and of PLC-γ (asterisk) are as indicated.
Figure 10
M-CSF–dependent association of PLC-γ2 and PYK2 in osteoclasts. Src+/? pOCs were cultured on Vn-coated dishes for 60 min in the absence of serum. (A) Total cell lysates were immunoprecipitated (IP) with anti–PLC-γ2 (lane 1) and anti–PLC-γ1 (lane 2), followed by blotting with anti-PYK2 (left), anti–PLC-γ2 (middle), or anti–PLC-γ1 (right) antibodies. (B) Lysates were immunoprecipitated with anti-PYK2 mAb 11 (lane 1) and anti-PYK2 N-19 antibodies (lane 2), followed by blotting with anti–PLC-γ2 (left) or anti-PYK2 (right). (C) Src+/? pOCs were cultured on PL or Vn for 60 min with or without 1 μM U73122. Lysates were immunoprecipitated with anti–PLC-γ2 and blotted with anti-PYK2 (left), and anti–PLC-γ2 (right) antibodies. (D) Lysates of Src+/? OCLs were incubated with GST fusion proteins containing NH2- and COOH-terminal SH2 domains or SH3 domains of PLC-γ1 and blotted with anti-PYK2 antibodies (top) or incubated with GST fusion proteins of NH2- or COOH-terminal domains or kinase (K) domain of PYK2 and blotted with anti–PLC-γ2 antibodies (bottom). (E) Src−/− pOCs (1.5 × 106 cells per condition) were plated on Vn and treated without and with M-CSF. Adhesion of Src+/? pOCs on Vn was used as control. Lysates were immunoprecipitated with anti–PLC-γ2 and blotted with anti-PYK2, followed by anti–PLC-γ2 antibodies (left) or immunoprecipitated with anti-N terminal PYK2 and blotted with anti–PLC-γ2, followed by anti-PYK2 antibodies (right). Molecular weight markers (in kD) are as indicated on the left. Positions of PYK2 (arrowhead) and of PLC-γ (asterisk) are as indicated.
Figure 10
M-CSF–dependent association of PLC-γ2 and PYK2 in osteoclasts. Src+/? pOCs were cultured on Vn-coated dishes for 60 min in the absence of serum. (A) Total cell lysates were immunoprecipitated (IP) with anti–PLC-γ2 (lane 1) and anti–PLC-γ1 (lane 2), followed by blotting with anti-PYK2 (left), anti–PLC-γ2 (middle), or anti–PLC-γ1 (right) antibodies. (B) Lysates were immunoprecipitated with anti-PYK2 mAb 11 (lane 1) and anti-PYK2 N-19 antibodies (lane 2), followed by blotting with anti–PLC-γ2 (left) or anti-PYK2 (right). (C) Src+/? pOCs were cultured on PL or Vn for 60 min with or without 1 μM U73122. Lysates were immunoprecipitated with anti–PLC-γ2 and blotted with anti-PYK2 (left), and anti–PLC-γ2 (right) antibodies. (D) Lysates of Src+/? OCLs were incubated with GST fusion proteins containing NH2- and COOH-terminal SH2 domains or SH3 domains of PLC-γ1 and blotted with anti-PYK2 antibodies (top) or incubated with GST fusion proteins of NH2- or COOH-terminal domains or kinase (K) domain of PYK2 and blotted with anti–PLC-γ2 antibodies (bottom). (E) Src−/− pOCs (1.5 × 106 cells per condition) were plated on Vn and treated without and with M-CSF. Adhesion of Src+/? pOCs on Vn was used as control. Lysates were immunoprecipitated with anti–PLC-γ2 and blotted with anti-PYK2, followed by anti–PLC-γ2 antibodies (left) or immunoprecipitated with anti-N terminal PYK2 and blotted with anti–PLC-γ2, followed by anti-PYK2 antibodies (right). Molecular weight markers (in kD) are as indicated on the left. Positions of PYK2 (arrowhead) and of PLC-γ (asterisk) are as indicated.
Figure 10
M-CSF–dependent association of PLC-γ2 and PYK2 in osteoclasts. Src+/? pOCs were cultured on Vn-coated dishes for 60 min in the absence of serum. (A) Total cell lysates were immunoprecipitated (IP) with anti–PLC-γ2 (lane 1) and anti–PLC-γ1 (lane 2), followed by blotting with anti-PYK2 (left), anti–PLC-γ2 (middle), or anti–PLC-γ1 (right) antibodies. (B) Lysates were immunoprecipitated with anti-PYK2 mAb 11 (lane 1) and anti-PYK2 N-19 antibodies (lane 2), followed by blotting with anti–PLC-γ2 (left) or anti-PYK2 (right). (C) Src+/? pOCs were cultured on PL or Vn for 60 min with or without 1 μM U73122. Lysates were immunoprecipitated with anti–PLC-γ2 and blotted with anti-PYK2 (left), and anti–PLC-γ2 (right) antibodies. (D) Lysates of Src+/? OCLs were incubated with GST fusion proteins containing NH2- and COOH-terminal SH2 domains or SH3 domains of PLC-γ1 and blotted with anti-PYK2 antibodies (top) or incubated with GST fusion proteins of NH2- or COOH-terminal domains or kinase (K) domain of PYK2 and blotted with anti–PLC-γ2 antibodies (bottom). (E) Src−/− pOCs (1.5 × 106 cells per condition) were plated on Vn and treated without and with M-CSF. Adhesion of Src+/? pOCs on Vn was used as control. Lysates were immunoprecipitated with anti–PLC-γ2 and blotted with anti-PYK2, followed by anti–PLC-γ2 antibodies (left) or immunoprecipitated with anti-N terminal PYK2 and blotted with anti–PLC-γ2, followed by anti-PYK2 antibodies (right). Molecular weight markers (in kD) are as indicated on the left. Positions of PYK2 (arrowhead) and of PLC-γ (asterisk) are as indicated.
Figure 10
M-CSF–dependent association of PLC-γ2 and PYK2 in osteoclasts. Src+/? pOCs were cultured on Vn-coated dishes for 60 min in the absence of serum. (A) Total cell lysates were immunoprecipitated (IP) with anti–PLC-γ2 (lane 1) and anti–PLC-γ1 (lane 2), followed by blotting with anti-PYK2 (left), anti–PLC-γ2 (middle), or anti–PLC-γ1 (right) antibodies. (B) Lysates were immunoprecipitated with anti-PYK2 mAb 11 (lane 1) and anti-PYK2 N-19 antibodies (lane 2), followed by blotting with anti–PLC-γ2 (left) or anti-PYK2 (right). (C) Src+/? pOCs were cultured on PL or Vn for 60 min with or without 1 μM U73122. Lysates were immunoprecipitated with anti–PLC-γ2 and blotted with anti-PYK2 (left), and anti–PLC-γ2 (right) antibodies. (D) Lysates of Src+/? OCLs were incubated with GST fusion proteins containing NH2- and COOH-terminal SH2 domains or SH3 domains of PLC-γ1 and blotted with anti-PYK2 antibodies (top) or incubated with GST fusion proteins of NH2- or COOH-terminal domains or kinase (K) domain of PYK2 and blotted with anti–PLC-γ2 antibodies (bottom). (E) Src−/− pOCs (1.5 × 106 cells per condition) were plated on Vn and treated without and with M-CSF. Adhesion of Src+/? pOCs on Vn was used as control. Lysates were immunoprecipitated with anti–PLC-γ2 and blotted with anti-PYK2, followed by anti–PLC-γ2 antibodies (left) or immunoprecipitated with anti-N terminal PYK2 and blotted with anti–PLC-γ2, followed by anti-PYK2 antibodies (right). Molecular weight markers (in kD) are as indicated on the left. Positions of PYK2 (arrowhead) and of PLC-γ (asterisk) are as indicated.
Similar articles
- Distinct roles of p130Cas and c-Cbl in adhesion-induced or macrophage colony-stimulating factor-mediated signaling pathways in prefusion osteoclasts.
Nakamura I, Rodan GA, Duong LT. Nakamura I, et al. Endocrinology. 2003 Nov;144(11):4739-41. doi: 10.1210/en.2003-0615. Epub 2003 Aug 13. Endocrinology. 2003. PMID: 12959979 - Abnormal localisation and hyperclustering of (alpha)(V)(beta)(3) integrins and associated proteins in Src-deficient or tyrphostin A9-treated osteoclasts.
Lakkakorpi PT, Nakamura I, Young M, Lipfert L, Rodan GA, Duong LT. Lakkakorpi PT, et al. J Cell Sci. 2001 Jan;114(Pt 1):149-160. doi: 10.1242/jcs.114.1.149. J Cell Sci. 2001. PMID: 11112699 - M-CSF induces the stable interaction of cFms with alphaVbeta3 integrin in osteoclasts.
Elsegood CL, Zhuo Y, Wesolowski GA, Hamilton JA, Rodan GA, Duong LT. Elsegood CL, et al. Int J Biochem Cell Biol. 2006;38(9):1518-29. doi: 10.1016/j.biocel.2006.02.011. Epub 2006 Mar 2. Int J Biochem Cell Biol. 2006. PMID: 16600665 - Role of c-Src in cellular events associated with colony-stimulating factor-1-induced spreading in osteoclasts.
Insogna K, Tanaka S, Neff L, Horne W, Levy J, Baron R. Insogna K, et al. Mol Reprod Dev. 1997 Jan;46(1):104-8. doi: 10.1002/(SICI)1098-2795(199701)46:1<104::AID-MRD16>3.0.CO;2-2. Mol Reprod Dev. 1997. PMID: 8981371 Review. - Integrin-mediated signaling in the regulation of osteoclast adhesion and activation.
Duong LT, Rodan GA. Duong LT, et al. Front Biosci. 1998 Aug 1;3:d757-68. doi: 10.2741/A319. Front Biosci. 1998. PMID: 9682033 Review.
Cited by
- Involvement of alpha(v)beta3 integrins in osteoclast function.
Nakamura I, Duong LT, Rodan SB, Rodan GA. Nakamura I, et al. J Bone Miner Metab. 2007;25(6):337-44. doi: 10.1007/s00774-007-0773-9. Epub 2007 Oct 25. J Bone Miner Metab. 2007. PMID: 17968485 Review. - Selective regulation of osteoclast adhesion and spreading by PLCγ/PKCα-PKCδ/RhoA-Rac1 signaling.
Kim JM, Lee K, Jeong D. Kim JM, et al. BMB Rep. 2018 May;51(5):230-235. doi: 10.5483/bmbrep.2018.51.5.198. BMB Rep. 2018. PMID: 29301608 Free PMC article. - Hydraulic Pressure during Fluid Flow Regulates Purinergic Signaling and Cytoskeleton Organization of Osteoblasts.
Gardinier JD, Gangadharan V, Wang L, Duncan RL. Gardinier JD, et al. Cell Mol Bioeng. 2014 Jun 1;7(2):266-277. doi: 10.1007/s12195-014-0329-8. Cell Mol Bioeng. 2014. PMID: 24910719 Free PMC article. - Comprehensive profiling analysis of actively resorbing osteoclasts identifies critical signaling pathways regulated by bone substrate.
Purdue PE, Crotti TN, Shen Z, Swantek J, Li J, Hill J, Hanidu A, Dimock J, Nabozny G, Goldring SR, McHugh KP. Purdue PE, et al. Sci Rep. 2014 Dec 23;4:7595. doi: 10.1038/srep07595. Sci Rep. 2014. PMID: 25534583 Free PMC article. - Dynamic changes in the osteoclast cytoskeleton in response to growth factors and cell attachment are controlled by beta3 integrin.
Faccio R, Novack DV, Zallone A, Ross FP, Teitelbaum SL. Faccio R, et al. J Cell Biol. 2003 Aug 4;162(3):499-509. doi: 10.1083/jcb.200212082. J Cell Biol. 2003. PMID: 12900398 Free PMC article.
References
- Asselin J., Gibbins J.M., Achison M., Lee Y.H., Morton L.F., Farndale R.W., Barnes M.J., Watson S. A collagen-like peptide stimulates tyrosine phosphorylation of Syk and phospholipase-Cγ2 in platelets independent of the integrin α2β1 . Blood. 1997;89:1235–1242. - PubMed
- Chen Q., Kinch M., Lin T., Burridge K., Juliano R. Integrin-mediated cell adhesion activates mitogen-activated protein kinases. J. Biol. Chem. 1994;269:26602–26605. - PubMed
- Czech M.P. PIP2 and PIP3complex roles at the cell surface. Cell. 2000;100:603–606. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous