Activation of the Drosophila NF-kappaB factor Relish by rapid endoproteolytic cleavage - PubMed (original) (raw)
Activation of the Drosophila NF-kappaB factor Relish by rapid endoproteolytic cleavage
S Stöven et al. EMBO Rep. 2000 Oct.
Abstract
The Rel/NF-kappaB transcription factor Relish plays a key role in the humoral immune response in Drosophila. We now find that activation of this innate immune response is preceded by rapid proteolytic cleavage of Relish into two parts. An N-terminal fragment, containing the DNA-binding Rel homology domain, translocates to the nucleus where it binds to the promoter of the Cecropin A1 gene and probably to the promoters of other antimicrobial peptide genes. The C-terminal IkappaB-like fragment remains in the cytoplasm. This endoproteolytic cleavage does not involve the proteasome, requires the DREDD caspase, and is different from previously described mechanisms for Rel factor activation.
Figures
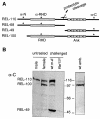
Fig. 1. Relish-derived proteins. (A) Map of proteins produced from the Relish gene, including the cleavage products REL-68 and REL-49. Positions of peptides used to raise specific antibodies are indicated by asterisks. (B) Western blot analysis of crude extracts from wild-type embryos, male and female flies and the Relish mutant Rel E20, detected with α-C. Where indicated, flies have been injected with E. cloacae for 6 h. Molecular weight markers are given in kDa.
Fig. 2. Signal-induced processing of Relish. (A and B) Western blots show time courses (in minutes) of protein extracts from either induced mbn-2 cells [(A), 25 µg protein/lane] or infected wild-type larvae [(B), ∼0.5 animal equivalent/lane]. In (A) the membrane was stripped before the second detection. Antibodies used to detect different forms of Relish are indicated to the left of each membrane. (C) Pulse–chase of in vivo labeled Relish-derived proteins. [35S]methionine-labeled proteins were extracted from mbn-2 cells, either induced with LPS for 5 min or left untreated. Relish products were immunoprecipitated with the indicated rabbit antibody. The samples were separated by gel electrophoresis, the gel dried and exposed to X-ray film. (D) Immunoblot of extracts from mbn-2 cells treated with cycloheximide to inhibit protein biosynthesis prior to and during the challenge.
Fig. 3. Subcellular localization of Relish before and after an immune challenge. (A) Western blot with cytoplasmic and nuclear extracts from mbn-2 cells (50 µg protein/lane), developed with α-C, stripped and then developed with α-RHD. (B) Immunostaining of mbn-2 cells with α-RHD and α-C, untreated or 30 s after challenge. (C) Fatbody from wild-type larvae, untreated or taken 1 h after infection, immunostained with α-RHD. Insets in (B) and (C) show DAPI staining as overlays from the same sample area. (D) Gel shift assay for involvement of Relish in the κB binding activity. Mbn-2 cells were induced for 1 h and nuclear extracts were incubated with CecA1 κB oligonucleotide. Where indicated, an anti-Relish antiserum or the corresponding preimmune serum (Pi) was added (left panel). In a competition experiment we also added increasing amounts of the peptide, against which the serum was raised. Addition of 500 ng of peptide without antibody did not cause any effect.
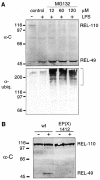
Fig. 4. Proteasome inhibition and Relish processing. (A) Immunoblotting of protein extracts from cell cultures, treated with different concentrations of MG132 prior to an immune challenge. The membrane was first developed with α-C and then with an anti-ubiquitin antibody to visualize the accumulation of polyubiquitylated proteins, indicated by the bracket. Addition of the solvent DMSO or of the inhibitors alone did not induce Relish processing. (B) Western blot with protein extracts from wild-type and EP(X)1412 third instar larvae. Where indicated (+) the animals have been infected 45 min before extract preparation. Different forms of Relish were detected with α-C.
Similar articles
- Caspase-mediated processing of the Drosophila NF-kappaB factor Relish.
Stoven S, Silverman N, Junell A, Hedengren-Olcott M, Erturk D, Engstrom Y, Maniatis T, Hultmark D. Stoven S, et al. Proc Natl Acad Sci U S A. 2003 May 13;100(10):5991-6. doi: 10.1073/pnas.1035902100. Epub 2003 May 5. Proc Natl Acad Sci U S A. 2003. PMID: 12732719 Free PMC article. - An in vitro study of NF-κB factors cooperatively in regulation of Drosophila melanogaster antimicrobial peptide genes.
Chowdhury M, Zhang J, Xu XX, He Z, Lu Y, Liu XS, Wang YF, Yu XQ. Chowdhury M, et al. Dev Comp Immunol. 2019 Jun;95:50-58. doi: 10.1016/j.dci.2019.01.017. Epub 2019 Feb 6. Dev Comp Immunol. 2019. PMID: 30735676 - The N-terminal half of the Drosophila Rel/NF-kappaB factor Relish, REL-68, constitutively activates transcription of specific Relish target genes.
Wiklund ML, Steinert S, Junell A, Hultmark D, Stöven S. Wiklund ML, et al. Dev Comp Immunol. 2009 May;33(5):690-6. doi: 10.1016/j.dci.2008.12.002. Epub 2009 Jan 9. Dev Comp Immunol. 2009. PMID: 19135474 - NF-κB/Rel proteins and the humoral immune responses of Drosophila melanogaster.
Ganesan S, Aggarwal K, Paquette N, Silverman N. Ganesan S, et al. Curr Top Microbiol Immunol. 2011;349:25-60. doi: 10.1007/82_2010_107. Curr Top Microbiol Immunol. 2011. PMID: 20852987 Free PMC article. Review. - Control of development and immunity by rel transcription factors in Drosophila.
Govind S. Govind S. Oncogene. 1999 Nov 22;18(49):6875-87. doi: 10.1038/sj.onc.1203223. Oncogene. 1999. PMID: 10602463 Review.
Cited by
- The Role of the Phylogenetically Conserved Cochaperone Protein Droj2/DNAJA3 in NF-κB Signaling.
Momiuchi Y, Kumada K, Kuraishi T, Takagaki T, Aigaki T, Oshima Y, Kurata S. Momiuchi Y, et al. J Biol Chem. 2015 Sep 25;290(39):23816-25. doi: 10.1074/jbc.M115.664193. Epub 2015 Aug 5. J Biol Chem. 2015. PMID: 26245905 Free PMC article. - Many cuts to ruin: a comprehensive update of caspase substrates.
Fischer U, Jänicke RU, Schulze-Osthoff K. Fischer U, et al. Cell Death Differ. 2003 Jan;10(1):76-100. doi: 10.1038/sj.cdd.4401160. Cell Death Differ. 2003. PMID: 12655297 Free PMC article. Review. - NF-kappaB in the immune response of Drosophila.
Hetru C, Hoffmann JA. Hetru C, et al. Cold Spring Harb Perspect Biol. 2009 Dec;1(6):a000232. doi: 10.1101/cshperspect.a000232. Epub 2009 Oct 7. Cold Spring Harb Perspect Biol. 2009. PMID: 20457557 Free PMC article. Review. - Current Status of Immune Deficiency Pathway in Tenebrio molitor Innate Immunity.
Jang HA, Kojour MAM, Patnaik BB, Han YS, Jo YH. Jang HA, et al. Front Immunol. 2022 Jul 4;13:906192. doi: 10.3389/fimmu.2022.906192. eCollection 2022. Front Immunol. 2022. PMID: 35860244 Free PMC article. Review. - Caspar, an adapter for VAPB and TER94, modulates the progression of ALS8 by regulating IMD/NFκB-mediated glial inflammation in a Drosophila model of human disease.
Tendulkar S, Hegde S, Garg L, Thulasidharan A, Kaduskar B, Ratnaparkhi A, Ratnaparkhi GS. Tendulkar S, et al. Hum Mol Genet. 2022 Aug 25;31(17):2857-2875. doi: 10.1093/hmg/ddac076. Hum Mol Genet. 2022. PMID: 35377453 Free PMC article.
References
- Chan Y.-M. and Jan, Y.N. (1998) Roles for proteolysis and trafficking in Notch maturation and signal transduction. Cell, 94, 423–426. - PubMed
- Chen P., Rodriguez, A., Erskine, R., Thach, T. and Abrams, J.M. (1998) Dredd, a novel effector of the apoptosis activators Reaper, Grim and Hid in Drosophila. Dev. Biol., 201, 202–216. - PubMed
- Edwards D.N., Towb, P. and Wasserman, S.A. (1997) An activity-dependent network of interactions links the Rel protein Dorsal with its cytoplasmic regulators. Development, 124, 3855–3864. - PubMed
- Elrod-Erickson M., Mishra, S. and Schneider, D. (2000) Interactions between the cellular and humoral immune responses in Drosophila. Curr. Biol., 10, 781–784. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous