Cytoplasmic RNA Polymerase in Escherichia coli - PubMed (original) (raw)
Cytoplasmic RNA Polymerase in Escherichia coli
N Shepherd et al. J Bacteriol. 2001 Apr.
Abstract
To obtain an estimate for the concentration of free functional RNA polymerase in the bacterial cytoplasm, the content of RNA polymerase beta and beta' subunits in DNA-free minicells from the minicell-producing Escherichia coli strain chi925 was determined. In bacteria grown in Luria-Bertani medium at 2.5 doublings/h, 1.0% of the total protein was RNA polymerase. The concentration of cytoplasmic RNA polymerase beta and beta' subunits in minicells produced by this strain corresponded to about 17% (or 2.5 microM) of the value found in whole cells. Literature data suggest that a similar portion of cytoplasmic RNA polymerase subunits is in RNA polymerase assembly intermediates and imply that free functional RNA polymerase can form a small percentage of the total functional enzyme in the cell. On infection with bacteriophage T7, 20% of the minicells produced progeny phage, whereas infection in 80% of the cells was abortive. RNA polymerase subunits in lysozyme-freeze-thaw lysates of minicells were associated with minicell envelopes and were without detectable activity in an in vitro transcription assay. Together, these results suggest that most functional RNA polymerase is associated with the DNA and that little if any segregates into DNA-free minicells.
Figures
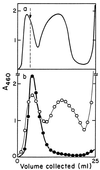
FIG. 1
Purification of minicells by zone sedimentation through sucrose gradients. (a) Sedimentation distribution obtained from a 100-ml culture after removal of the major portion of large cells by differential centrifugation. (b) The separately pooled minicell (left of dashed line) and small-cell (right of dashed line) preparations from the first centrifugation were concentrated by centrifugation (15,000 rpm for 30 min in a Sorvall SS34 rotor), layered onto seperate sucrose gradients, and centrifuged as before. ●, minicell fraction; ○, small-cell fraction.
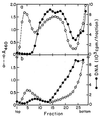
FIG. 2
Location of DNA in sedimentation distributions of minicells without and with plasmid R6K. Two 100-ml cultures of χ925 (a) and χ925 carrying the R6K plasmid (b) were grown for 10 to 12 h in supplemented medium C containing 250 μg of deoxyadenosine per ml and 0.1 μCi of [6-3H]thymidine per ml (28 Ci/mmol). At an OD460 of 1.3 to 1.7, the cultures were centrifuged, and the crude minicell preparations obtained by differential centrifugation were analyzed by zone sedimentation through sucrose gradients: ○, OD460; ●, acid-precipitable radioactivity. For gradients containing plasmid-carrying χ925 cells, the small cell peak is missing, possibly due to cell aggregation caused by sex pili (see Materials and Methods).
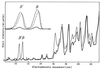
FIG. 3
RNA polymerase β and β′ subunits in minicells, small cells, and large cells. Densitometer tracing was performed on Coomassie blue-stained polypeptides after electrophoresis. Traces are shown for minicells (thick line), small cells (dashed line), and large cells (thin line). The insert illustrates an expanded abscissa of the ββ′ region. Each sample slot in the SDS-gel contained 0.30 mg of protein. Measurement of the area under a given peak results in an underestimate of the amount of protein present in the smallest and largest peaks; corrections for this were made as described in Materials and Methods.
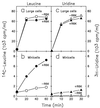
FIG. 4
RNA and protein synthesis in minicells and large cells. The incorporation of [14C]leucine or [3H]uridine into acid-precipitable material was measured in large cells (a and c) or minicells (b and d) of strain χ925 with (●, ▴) and without (○, ▵) R6K plasmid. Purified minicells and large cells were suspended to an OD460 of 0.4 in medium C supplemented with 0.2% glucose, 320 μg of
dl
-threonine per ml, and 20 μg of thiamine per ml. Each cell suspension was divided into equal portions for RNA and protein labeling. The portion for RNA labeling received 270 μg of
l
-leucine per ml. The cell suspensions were incubated at 37°C for 10 min with aeration before addition of radioctivity and plating for viable cells. For incorporation of [14C]leucine (a and b), a 2-ml portion of minicells or large cells, respectively, was added to 0.2 ml (0.1 μCi) of [14C]leucine (312 Ci/mol) at time zero and incubated at 37°C with aeration. Samples (0.1 ml) were removed into 1 ml of cold 1 M TCA, heated for 30 s in a boiling-water bath, and cooled to 0°C. Radioactive acid-precipitable material was collected on glass fiber filters and counted. For incorporation of [3H]uridine, a 2.5-ml portion of minicells or large cells was added to 0.5 ml (2.5 μCi) of [5-3H]uridine (21 Ci/mmol) at time zero and incubated at 37°C with aeration. Samples (0.5 ml) were added to 2 ml of cold 1 M TCA, and acid-precipitable radioactivity was collected on glass fiber filters and counted. The scales for large cells and minicells differ by 10-fold. The time zero values represent background radioactivity, and the slight increases in radioactivity seen with plasmid-free minicells are presumed to result from contaminating nucleated cells. The labeling plateaus observed with large cells reflect exhaustion of the exogenous precursor from the medium.
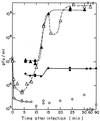
FIG. 5
Adsorption and one-step growth curve of T7 bacteriophage infection of minicells and large cells of strain χ925. Purified minicells and large cells were resuspended in supplemented LB medium to an OD460 of 0.455 (about 8 ×108 minicells and 3 ×105 viable cells/ml) and 0.480 (8 ×107 viable cells/ml), respectively. The minicells (2.7 ml) were incubated for 5 min at 37°C with aeration before the addition at t = 0 of 0.3 ml of T7 phage to a final concentration of 1.3 ×107 PFU/ml. ○, adsorption to minicells. (At the indicated times, 0.1-ml samples were diluted 1:50 into chloroform-saturated supplemented LB medium at 37°C, and the number of free phage in these chloroform tubes was later determined by plating). ●, one-step growth curve for minicells, determined by removing 0.1-ml samples from the absorption tube at various times, making appropriate dilutions, and plating. To prevent readsorption of progeny phage, samples were removed from a tube (made at t = 8.5 min) containing a 1:250 dilution of the adsorption mixture. ▵, adsorption kinetics; ▴, one-step growth curve with χ925 large cells, obtained as with minicells, except for different dilutions.
Similar articles
- Transcription of plasmid DNA in Escherichia coli minicells.
Crooks JH, Ullman M, Zoller M, Levy SB. Crooks JH, et al. Plasmid. 1983 Jul;10(1):66-72. doi: 10.1016/0147-619x(83)90058-6. Plasmid. 1983. PMID: 6194538 - Bacteriophage T7 DNA ejection into cells is initiated by an enzyme-like mechanism.
Kemp P, Gupta M, Molineux IJ. Kemp P, et al. Mol Microbiol. 2004 Aug;53(4):1251-65. doi: 10.1111/j.1365-2958.2004.04204.x. Mol Microbiol. 2004. PMID: 15306026 - A structure/function analysis of Escherichia coli RNA polymerase.
Gross CA, Chan CL, Lonetto MA. Gross CA, et al. Philos Trans R Soc Lond B Biol Sci. 1996 Apr 29;351(1339):475-82. doi: 10.1098/rstb.1996.0045. Philos Trans R Soc Lond B Biol Sci. 1996. PMID: 8735269 Review. - Genome function--a virus-world view.
Yin J. Yin J. Adv Exp Med Biol. 2004;547:31-46. doi: 10.1007/978-1-4419-8861-4_4. Adv Exp Med Biol. 2004. PMID: 15230091 Review.
Cited by
- Mycobacterium tuberculosis RNA polymerase-binding protein A (RbpA) and its interactions with sigma factors.
Bortoluzzi A, Muskett FW, Waters LC, Addis PW, Rieck B, Munder T, Schleier S, Forti F, Ghisotti D, Carr MD, O'Hare HM. Bortoluzzi A, et al. J Biol Chem. 2013 May 17;288(20):14438-14450. doi: 10.1074/jbc.M113.459883. Epub 2013 Apr 2. J Biol Chem. 2013. PMID: 23548911 Free PMC article. - A study in molecular contingency: glutamine phosphoribosylpyrophosphate amidotransferase is a promiscuous and evolvable phosphoribosylanthranilate isomerase.
Patrick WM, Matsumura I. Patrick WM, et al. J Mol Biol. 2008 Mar 21;377(2):323-36. doi: 10.1016/j.jmb.2008.01.043. Epub 2008 Jan 26. J Mol Biol. 2008. PMID: 18272177 Free PMC article. - High-affinity quasi-specific sites in the genome: how the DNA-binding proteins cope with them.
Chakrabarti J, Chandra N, Raha P, Roy S. Chakrabarti J, et al. Biophys J. 2011 Sep 7;101(5):1123-9. doi: 10.1016/j.bpj.2011.07.041. Biophys J. 2011. PMID: 21889449 Free PMC article. - A superresolution census of RNA polymerase.
Klumpp S. Klumpp S. Biophys J. 2013 Dec 17;105(12):2613-4. doi: 10.1016/j.bpj.2013.11.018. Biophys J. 2013. PMID: 24359730 Free PMC article. No abstract available. - Developing a pathway-independent and full-autonomous global resource allocation strategy to dynamically switching phenotypic states.
Wu J, Bao M, Duan X, Zhou P, Chen C, Gao J, Cheng S, Zhuang Q, Zhao Z. Wu J, et al. Nat Commun. 2020 Nov 2;11(1):5521. doi: 10.1038/s41467-020-19432-2. Nat Commun. 2020. PMID: 33139748 Free PMC article.
References
- Baracchini E, Bremer H. Control of rRNA synthesis in Escherichia coli at increased rrn gene dosage. J Biol Chem. 1991;266:11753–11760. - PubMed
- Bremer H, Dennis P P. Modulation of chemical composition and other parameters of the cell by growth rate. In: Neidhardt F C, et al., editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: American Society for Microbiology; 1996. pp. 1553–1569.
- Bremer H, Ehrenberg M. Guanosine tetraphosphate as a global regulator of bacterial RNA synthesis: a model involving RNA polymerase pausing and queuing. Biochim Biophys Acta. 1995;1262:15–36. - PubMed
- Burgess R R, Travers A A, Dunn J J, Bautz E K F. Factor stimulating transcription hy RNA polymerase. Nature (London) 1969;221:43–46. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources