Biofilm formation by Staphylococcus epidermidis depends on functional RsbU, an activator of the sigB operon: differential activation mechanisms due to ethanol and salt stress - PubMed (original) (raw)
Biofilm formation by Staphylococcus epidermidis depends on functional RsbU, an activator of the sigB operon: differential activation mechanisms due to ethanol and salt stress
J K Knobloch et al. J Bacteriol. 2001 Apr.
Abstract
Staphylococcus epidermidis is a common pathogen in medical device-associated infections. Its major pathogenetic factor is the ability to form adherent biofilms. The polysaccharide intercellular adhesin (PIA), which is synthesized by the products of the icaADBC gene cluster, is essential for biofilm accumulation. In the present study, we characterized the gene locus inactivated by Tn917 insertions of two isogenic, icaADBC-independent, biofilm-negative mutants, M15 and M19, of the biofilm-producing bacterium S. epidermidis 1457. The insertion site was the same in both of the mutants and was located in the first gene, rsbU, of an operon highly homologous to the sigB operons of Staphylococcus aureus and Bacillus subtilis. Supplementation of Trypticase soy broth with NaCl (TSB(NaCl)) or ethanol (TSB(EtOH)), both of which are known activators of sigB, led to increased biofilm formation and PIA synthesis by S. epidermidis 1457. Insertion of Tn917 into rsbU, a positive regulator of alternative sigma factor sigma(B), led to a biofilm-negative phenotype and almost undetectable PIA production. Interestingly, in TSB(EtOH), the mutants were enabled to form a biofilm again with phenotypes similar to those of the wild type. In TSB(NaCl), the mutants still displayed a biofilm-negative phenotype. No difference in primary attachment between the mutants and the wild type was observed. Similar phenotypic changes were observed after transfer of the Tn917 insertion of mutant M15 to the independent and biofilm-producing strain S. epidermidis 8400. In 11 clinical S. epidermidis strains, a restriction fragment length polymorphism of the sigB operon was detected which was independent of the presence of the icaADBC locus and a biofilm-positive phenotype. Obviously, different mechanisms are operative in the regulation of PIA expression in stationary phase and under stress induced by salt or ethanol.
Figures
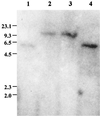
FIG. 1
Southern blot of _Eco_RI-digested chromosomal DNA using a 32P-labeled S. epidermidis sigB PCR fragment from pJKMK401 as a probe. Lanes 1, S. epidermidis 1457; 2, S. epidermidis M15; 3, S. epidermidis M19; 4, S. epidermidis M12 (class II mutant). The values on the left are sizes in kilobases.
FIG. 2
Comparison of the organization of the sigB operon of S. epidermidis 1457 and that of the sigB operons of S. aureus and B. subtilis. In the physical maps, genes are indicated as arrows. Homology of nucleotide (NT) sequences and the identity and similarity of the deduced amino acid (AA) sequences between corresponding genes are shown. The positions of putative promoters are indicated. The Tn_917_ insertion site of mutants M15 and M19 is indicated. The transcriptional direction of the erm gene of Tn_917_ is shown by an arrowhead. The typical 5-nt duplication at the Tn_917_ insertion site is in boldface.
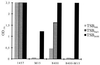
FIG. 3
Biofilm formation under different stress conditions. S. epidermidis 1457, isogenic mutant M15, independent wild-type S. epidermidis 8400, and its transductant 8400-M15 were analyzed using TSBBBL, TSBNaCl, and TSBEtOH as the growth media. Results of a representative experiment are shown.
FIG. 4
Primary attachment of S. epidermidis 1457 (●) and isogenic mutant M15 (▾) grown in TSBBBL (A), TSBNaCl (B), or TSBEtOH (C) to polystyrene cell culture plates (Nunclon Delta; Nunc). Bacteria were inoculated into the plates at various concentrations and incubated for 1 h at 37°C. Attached bacterial cells were detected by ELISA as described in Materials and Methods. Results of a representative experiment are shown.
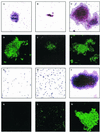
FIG. 5
Cell cluster formation and PIA expression by S. epidermidis 1457 and mutant M15 under different stress conditions. S. epidermidis 1457 (A to F) and isogenic mutant M15 (G to M) were grown in tissue culture plates as a biofilm in TSBBBL (A, D, G, and K), TSBNaCl (B, E, H, and L), or TSBEtOH (C, F, I, and M) for 22 h at 37°C. Cells were scraped from the surface, and appropriate dilutions in phosphate-buffered saline were applied to microscope slides or immunofluorescence slides. Microphotographs of representative fields are shown after Gram staining (A to C and G to I) or after IFA using a PIA-specific antiserum as described in Materials and Methods (D to F and K to M). Results of a representative experiment are shown.
FIG. 6
sigB RFLP of different clinical S. epidermidis isolates. PCR fragments containing almost the complete sigB operon were cleaved with _Hin_fI. Two different sigB RFLP types, A and B, were detected. The S. epidermidis strains which display sigB RFLP type A were 1457 (lane 1), RP62A (lane 2), 521 (lane 4), 1057 (lane 5), 10333 (lane 6), 9225 (lane 9), and 939 (lane 11). The S. epidermidis strains which display sigB RFLP type B were SE5 (lane 3), 5179 (lane 7), 7837 (lane 8), and 9896 (lane 10).
Similar articles
- Inactivations of rsbU and sarA by IS256 represent novel mechanisms of biofilm phenotypic variation in Staphylococcus epidermidis.
Conlon KM, Humphreys H, O'Gara JP. Conlon KM, et al. J Bacteriol. 2004 Sep;186(18):6208-19. doi: 10.1128/JB.186.18.6208-6219.2004. J Bacteriol. 2004. PMID: 15342591 Free PMC article. - The role of sigmaB in persistence of Staphylococcus epidermidis foreign body infection.
Pintens V, Massonet C, Merckx R, Vandecasteele S, Peetermans WE, Knobloch JK, Van Eldere J. Pintens V, et al. Microbiology (Reading). 2008 Sep;154(Pt 9):2827-2836. doi: 10.1099/mic.0.2007/015768-0. Microbiology (Reading). 2008. PMID: 18757816 - Polysaccharide intercellular adhesin in biofilm: structural and regulatory aspects.
Arciola CR, Campoccia D, Ravaioli S, Montanaro L. Arciola CR, et al. Front Cell Infect Microbiol. 2015 Feb 10;5:7. doi: 10.3389/fcimb.2015.00007. eCollection 2015. Front Cell Infect Microbiol. 2015. PMID: 25713785 Free PMC article. Review. - ica and beyond: biofilm mechanisms and regulation in Staphylococcus epidermidis and Staphylococcus aureus.
O'Gara JP. O'Gara JP. FEMS Microbiol Lett. 2007 May;270(2):179-88. doi: 10.1111/j.1574-6968.2007.00688.x. Epub 2007 Apr 10. FEMS Microbiol Lett. 2007. PMID: 17419768 Review.
Cited by
- The Transcription Factor SpoVG Is of Major Importance for Biofilm Formation of Staphylococcus epidermidis under In Vitro Conditions, but Dispensable for In Vivo Biofilm Formation.
Benthien H, Fresenborg B, Pätzold L, Elhawy MI, Huc-Brandt S, Beisswenger C, Krasteva-Christ G, Becker SL, Molle V, Knobloch JK, Bischoff M. Benthien H, et al. Int J Mol Sci. 2022 Mar 17;23(6):3255. doi: 10.3390/ijms23063255. Int J Mol Sci. 2022. PMID: 35328675 Free PMC article. - Bacterial cell attachment, the beginning of a biofilm.
Palmer J, Flint S, Brooks J. Palmer J, et al. J Ind Microbiol Biotechnol. 2007 Sep;34(9):577-88. doi: 10.1007/s10295-007-0234-4. Epub 2007 Jul 6. J Ind Microbiol Biotechnol. 2007. PMID: 17619090 Review. - Detection of virulence-associated genes not useful for discriminating between invasive and commensal Staphylococcus epidermidis strains from a bone marrow transplant unit.
Rohde H, Kalitzky M, Kröger N, Scherpe S, Horstkotte MA, Knobloch JK, Zander AR, Mack D. Rohde H, et al. J Clin Microbiol. 2004 Dec;42(12):5614-9. doi: 10.1128/JCM.42.12.5614-5619.2004. J Clin Microbiol. 2004. PMID: 15583290 Free PMC article. - The ica operon and biofilm production in coagulase-negative Staphylococci associated with carriage and disease in a neonatal intensive care unit.
de Silva GD, Kantzanou M, Justice A, Massey RC, Wilkinson AR, Day NP, Peacock SJ. de Silva GD, et al. J Clin Microbiol. 2002 Feb;40(2):382-8. doi: 10.1128/JCM.40.02.382-388.2002. J Clin Microbiol. 2002. PMID: 11825946 Free PMC article. - Non-biofilm-forming commensal Staphylococcus epidermidis isolates produce biofilm in the presence of trypsin.
Martínez-García S, Ortega-Peña S, De Haro-Cruz MJ, Aguilera-Arreola MG, Alcántar-Curiel MD, Betanzos-Cabrera G, Jan-Roblero J, Pérez-Tapia SM, Rodríguez-Martínez S, Cancino-Diaz ME, Cancino-Diaz JC. Martínez-García S, et al. Microbiologyopen. 2019 Oct;8(10):e906. doi: 10.1002/mbo3.906. Epub 2019 Aug 7. Microbiologyopen. 2019. PMID: 31389671 Free PMC article.
References
- Alper S, Dufour A, Garsin D A, Duncan L, Losick R. Role of adenosine nucleotides in the regulation of a stress-response transcription factor in Bacillus subtilis. J Mol Biol. 1996;260:165–177. - PubMed
- Christensen G D, Baldassarri L, Simpson W A. Colonization of medical devices by coagulase-negative staphylococci. In: Bisno A L, Waldvogel F A, editors. Infections associated with indwelling medical devices. Washington, D.C.: American Society for Microbiology; 1994. pp. 45–78.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources