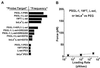Chemically distinct transition states govern rapid dissociation of single L-selectin bonds under force - PubMed (original) (raw)
Chemically distinct transition states govern rapid dissociation of single L-selectin bonds under force
E Evans et al. Proc Natl Acad Sci U S A. 2001.
Abstract
Carbohydrate--protein bonds interrupt the rapid flow of leukocytes in the circulation by initiation of rolling and tethering at vessel walls. The cell surface carbohydrate ligands are glycosylated proteins like the mucin P-selectin glycoprotein ligand-1 (PSGL-1), which bind ubiquitously to the family of E-, P-, and L-selectin proteins in membranes of leukocytes and endothelium. The current view is that carbohydrate-selectin bonds dissociate a few times per second, and the unbinding rate increases weakly with force. However, such studies have provided little insight into how numerous hydrogen bonds, a Ca(2+) metal ion bond, and other interactions contribute to the mechanical strength of these attachments. Decorating a force probe with very dilute ligands and controlling touch to achieve rare single-bond events, we have varied the unbinding rates of carbohydrate--selectin bonds by detachment with ramps of force/time from 10 to 100,000 pN/sec. Testing PSGL-1, its outer 19 aa (19FT), and sialyl Lewis(X) (sLe(X)) against L-selectin in vitro on glass microspheres and in situ on neutrophils, we found that the unbinding rates followed the same dependence on force and increased by nearly 1,000-fold as rupture forces rose from a few to approximately 200 pN. Plotted on a logarithmic scale of loading rate, the rupture forces reveal two prominent energy barriers along the unbinding pathway. Strengths above 75 pN arise from rapid detachment (<0.01 sec) impeded by an inner barrier that requires a Ca(2+) bond between a single sLe(X) and the lectin domain. Strengths below 75 pN occur under slow detachment (>0.01 sec) impeded by the outer barrier, which appears to involve an array of weak (putatively hydrogen) bonds.
Figures
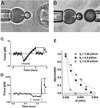
Figure 1
(A and B) With ≈2-μm glass spheres as tips decorated by a carbohydrate ligand, BFPs at the left in both images were kept stationary. The targets, a PMN at the right in_A_ and an L-selectin tethered sphere at the right in_B_, were translated to/from contact with the BFP at speeds from 1 to 50,000 nm/sec. The BFP spring was a pressurized red blood cell capsule (28). Controlled by micropipette suction, capsule membrane tension was preselected to set the force constant_k_f between 0.1 and 2 pN/nm. With ultrafast video processing (≈1,500 frames per sec), a simulated cursor (white dot between arrows) tracked movement of the BFP tip at a resolution of 5–7 nm. (C and D) Traces of BFP force over time for approach (touch under feed back control) and retraction of a PMN. The rise in force shows the loading up to the maximum BFP elastic force f_b at bond rupture. (C) A bond loaded slowly at ≈10 pN/sec held the tip to the surface for ≈1 sec and broke at_f_b ≈ 10 pN. (D) A bond loaded at ≈50,000 pN/sec held the tip to the surface for ≈0.003 sec and broke at f_b ≈ 170 pN. (E) BFP recovery at 0.00067-sec intervals after bond rupture for different values of stiffness_k_f; extension is normalized by the maximum BFP stretch at Δ_t = 0. Dotted lines show the decay defined by exp(−_k_fΔ_t/ζp) and the ratio of stiffness to the damping coefficient ζp = 0.00048 pN⋅sec/nm.
Figure 2
(A) Relative frequencies of attachment for carbohydrate:L-selectin interactions in Ca2+ (top four lanes on a scale of ≈1 per 10 touches), in the presence of blocking antibodies and Ca2+ or EDTA (middle five lanes), for nonspecific interactions with PEGylated spheres (bottom four lanes), all probed under the fixed conditions of impingement force and contact duration. (B) Nonspecific forces (±SD) measured ≈1 per 100 touches to PEGylated microspheres.
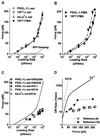
Figure 3
(A–C) The most frequent forces observed to rupture carbohydrate bonds to L-selectin as functions of loading rate. (The values are shown ± standard error in force, which represents the uncertainty in most frequent force; standard deviations in each force histogram were always comparable to the slope of the appropriate linear-like regime, as expected for single bond kinetics.) (A) Forces in Ca2+ for 19FT attachments to L-selectin in situ on PMNs and for PSGL-1, 19FT, bsLeX attachments to L-selectin in vitro on spheres. The lower dotted curve is the correction for viscous damping added to the elastic BFP force illustrated by the upper dotted curve. The solid curve is the continuous spectrum for passage of two energy barriers: an outer barrier at a distance_x_β ≈ 4Å along the direction of force with a transition rate 1/_t_off ≈ 3 per second and an inner barrier at a distance_x_β ≈ 0.6Å with a transition rate 1/_t_off ≈ 100 per sec. (B) Forces in Ca2+ for PSGL-1 and 19FT attachments to L-selectin in situ on PMNs. The solid curve is the spectrum in A; the dashed-dotted curve is the spectrum predicted for a zipper of two bonds, where each bond is governed by the solid-curve spectrum. (C) Forces in 10–20 mM EDTA for PSGL-1 and bsLeX attachments to L-selectin on spheres and for PSGL-1 attachments to L-selectin on spheres in Ca2+ blocked by mAbs KPL1, DREG55, and DREG200. The solid curve is the spectrum in A. (D) Kinetic profiles for rate of PSGL-1 dissociation from L-selectin under force in Ca2+ (solid curve) and in EDTA or blocked by KPL1 and DREG 55 (dotted curve). Also shown are rates of transient cell detachment observed at different video framing speeds, 30 per sec (9) and 240 per sec (16), in flow chamber studies.
Similar articles
- Mechanical switching and coupling between two dissociation pathways in a P-selectin adhesion bond.
Evans E, Leung A, Heinrich V, Zhu C. Evans E, et al. Proc Natl Acad Sci U S A. 2004 Aug 3;101(31):11281-6. doi: 10.1073/pnas.0401870101. Epub 2004 Jul 26. Proc Natl Acad Sci U S A. 2004. PMID: 15277675 Free PMC article. - Catch bonds govern adhesion through L-selectin at threshold shear.
Yago T, Wu J, Wey CD, Klopocki AG, Zhu C, McEver RP. Yago T, et al. J Cell Biol. 2004 Sep 13;166(6):913-23. doi: 10.1083/jcb.200403144. J Cell Biol. 2004. PMID: 15364963 Free PMC article. - The molecular mechanics of P- and L-selectin lectin domains binding to PSGL-1.
Rinko LJ, Lawrence MB, Guilford WH. Rinko LJ, et al. Biophys J. 2004 Jan;86(1 Pt 1):544-54. doi: 10.1016/S0006-3495(04)74133-8. Biophys J. 2004. PMID: 14695299 Free PMC article. - E-selectin ligands as mechanosensitive receptors on neutrophils in health and disease.
Chase SD, Magnani JL, Simon SI. Chase SD, et al. Ann Biomed Eng. 2012 Apr;40(4):849-59. doi: 10.1007/s10439-011-0507-y. Epub 2012 Jan 24. Ann Biomed Eng. 2012. PMID: 22271244 Free PMC article. Review. - For catch bonds, it all hinges on the interdomain region.
Thomas W. Thomas W. J Cell Biol. 2006 Sep 25;174(7):911-3. doi: 10.1083/jcb.200609029. J Cell Biol. 2006. PMID: 17000873 Free PMC article. Review.
Cited by
- Biophysical description of multiple events contributing blood leukocyte arrest on endothelium.
Robert P, Touchard D, Bongrand P, Pierres A. Robert P, et al. Front Immunol. 2013 May 15;4:108. doi: 10.3389/fimmu.2013.00108. eCollection 2013. Front Immunol. 2013. PMID: 23750158 Free PMC article. - A single molecule assay to probe monovalent and multivalent bonds between hyaluronan and its key leukocyte receptor CD44 under force.
Bano F, Banerji S, Howarth M, Jackson DG, Richter RP. Bano F, et al. Sci Rep. 2016 Sep 29;6:34176. doi: 10.1038/srep34176. Sci Rep. 2016. PMID: 27679982 Free PMC article. - Mechanically unfolding the small, topologically simple protein L.
Brockwell DJ, Beddard GS, Paci E, West DK, Olmsted PD, Smith DA, Radford SE. Brockwell DJ, et al. Biophys J. 2005 Jul;89(1):506-19. doi: 10.1529/biophysj.105.061465. Epub 2005 Apr 29. Biophys J. 2005. PMID: 15863479 Free PMC article. - Membrane-based actuation for high-speed single molecule force spectroscopy studies using AFM.
Sarangapani K, Torun H, Finkler O, Zhu C, Degertekin L. Sarangapani K, et al. Eur Biophys J. 2010 Jul;39(8):1219-27. doi: 10.1007/s00249-009-0575-1. Epub 2010 Jan 7. Eur Biophys J. 2010. PMID: 20054686 - Deformation-enhanced fluctuations in the red cell skeleton with theoretical relations to elasticity, connectivity, and spectrin unfolding.
Lee JC, Discher DE. Lee JC, et al. Biophys J. 2001 Dec;81(6):3178-92. doi: 10.1016/S0006-3495(01)75954-1. Biophys J. 2001. PMID: 11720984 Free PMC article.
References
- Lawrence M B, Springer T A. Cell. 1991;65:859–873. - PubMed
- Springer T A. Nature (London) 1990;346:425–434. - PubMed
- Springer T A. Cell. 1994;76:301–314. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI047294/AI/NIAID NIH HHS/United States
- R01 HL031579/HL/NHLBI NIH HHS/United States
- GM59100/GM/NIGMS NIH HHS/United States
- HL54700/HL/NHLBI NIH HHS/United States
- AI47294/AI/NIAID NIH HHS/United States
- HL31579/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous