Turning a disulfide isomerase into an oxidase: DsbC mutants that imitate DsbA - PubMed (original) (raw)
Turning a disulfide isomerase into an oxidase: DsbC mutants that imitate DsbA
M W Bader et al. EMBO J. 2001.
Abstract
There are two distinct pathways for disulfide formation in prokaryotes. The DsbA-DsbB pathway introduces disulfide bonds de novo, while the DsbC-DsbD pathway functions to isomerize disulfides. One of the key questions in disulfide biology is how the isomerase pathway is kept separate from the oxidase pathway in vivo. Cross-talk between these two systems would be mutually destructive. To force communication between these two systems we have selected dsbC mutants that complement a dsbA null mutation. In these mutants, DsbC is present as a monomer as compared with dimeric wild-type DsbC. Based on these findings we rationally designed DsbC mutants in the dimerization domain. All of these mutants are able to rescue the dsbA null phenotype. Rescue depends on the presence of DsbB, the native re-oxidant of DsbA, both in vivo and in vitro. Our results suggest that dimerization acts to protect DsbC's active sites from DsbB-mediated oxidation. These results explain how oxidative and reductive pathways can co-exist in the periplasm of Escherichia coli.
Figures
Fig. 1. Identification of two DsbC mutants that rescue the dsbA null phenotype. Colonies were stabbed onto M63 motility plates and grown for 24 h. A dsbA null mutation leads to a severe defect in disulfide bond formation causing the loss of motility (JCB817). This motility is not restored by the plasmid pMB69, which carries the wild-type dsbC gene. Two DsbC mutants that conferred motility were selected a number of times independently, following random mutagenesis of the dsbC gene. The two mutations were identified as dsbC G49R and G49E. The minimal medium contains 0.4% glycerol as carbon source and 0.2% arabinose to induce expression of DsbC under ara control. No rescue was observed in the absence of arabinose (data not shown).
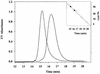
Fig. 2. DsbC G49R is a monomer. The DsbC mutant G49R was purified and analyzed by analytical gel filtration. DsbC G49R shows a clear difference in migration on a Sephadex 200 column as compared with wild-type DsbC. The inset shows a standard curve generated with proteins of known molecular weight (open circles): bovine serum albumin (66 kDa), carbonic anhydrase (29 kDa), cytochrome c (12.4 kDa) and apoprotinin (6.5 kDa). The apparent molecular weights of wild-type DsbC and DsbC G49R (filled triangles) were determined according to this curve. An apparent mol. wt of 31.6 kDa was calculated for DsbC G49R. Wild-type DsbC runs with an apparent mol. wt of 51.4 kDa. This suggests that G49R is monomeric.
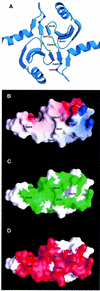
Fig. 3. Rational design of mutants in the dimerization domain of DsbC. (A) The crystal structure of DsbC is shown with the mutated residues represented as small spheres. The monomers interact via an N-terminal dimerization interface that consists of two very short consecutive β-strands from each monomer. Two extended β-sheets are formed, which consist of two strands from one monomer and four strands from the other. Two DsbC mutants that were found to complement a dsbA null mutant were mapped to G49 (drawn as a green sphere), which lies in between the interacting β-strands. These mutations replace G49 with either arginine or glutamate. An additional five residues, which were predicted to provide crucial interactions in the dimerization interface and were subject to mutagenesis, are also shown as small spheres. (B) Surface potential of DsbC’s dimerization interface. Only one monomer is shown. Values range from +7kT (blue) to 0 (white) to –7kT (red). M27, V54 and I47 were mutated to lysines. H45 forms a salt bridge with Asp53 on the other monomer (compare with Figure 3A), and was mutated to an aspartate. (C) Same as (B), except that the green color highlights conserved residues. Conserved residues were mapped according to the sequence alignment of 15 DsbC molecules. A BLAST search was performed with the entire DsbC protein sequence at the NCBI database of finished and unfinished bacterial genomes (
http://www.ncbi.nlm.nih.gov/Microb\_blast/unfinishedgenome.html
). Fifteen microbial sequences were aligned with CLUSTAL W (Thompson et al., 1994). Identical and similar residues that are found in at least 60% of the aligned sequences are shaded in green. (D) Molecular surface of the dimerization domain showing residues involved in dimer formation. Note that most of the residues involved in dimerization are conserved.
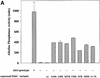
Fig. 4. (A) All mutant DsbCs promote folding of alkaline phosphatase in vivo. Plasmids encoding DsbC or DsbC variants, respectively, were transformed into JCB817 (_dsbA_–). The strains were grown in M63 media supplemented with 0.4% glycerol as carbon source and 0.2% arabinose to induce expression from the pBAD33 derived plasmids. The activity of alkaline phosphatase was determined by a standard assay described in Materials and methods. JCB816 (wild type) served as a positive control. The different plasmids are listed in Table II. (B) Rescue of the dsbA null phenotype strongly depends on the presence of DsbB. Conditions were essentially as in (A), except that JCB818 (_dsbA_–, _dsbB_–) served as a strain background.
Fig. 4. (A) All mutant DsbCs promote folding of alkaline phosphatase in vivo. Plasmids encoding DsbC or DsbC variants, respectively, were transformed into JCB817 (_dsbA_–). The strains were grown in M63 media supplemented with 0.4% glycerol as carbon source and 0.2% arabinose to induce expression from the pBAD33 derived plasmids. The activity of alkaline phosphatase was determined by a standard assay described in Materials and methods. JCB816 (wild type) served as a positive control. The different plasmids are listed in Table II. (B) Rescue of the dsbA null phenotype strongly depends on the presence of DsbB. Conditions were essentially as in (A), except that JCB818 (_dsbA_–, _dsbB_–) served as a strain background.
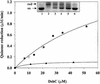
Fig. 5. DsbB re-oxidizes monomeric DsbC in vitro. DsbB’s ability to re-oxidize monomeric DsbC (H45D, filled circles; G49R, open circles) was measured by its ubiquinone reductase activity. The assay buffer consists of 100 mM sodium phosphate pH 6.0, 0.1% dodecyl-maltoside, 20 µM ubiquinone and 1 µM DsbB. The concentration of reduced DsbC H45D was steadily increased up to 50 µM. Activity of DsbB was plotted as quinone reductase activity. Reduced wild-type DsbC served as a control (filled triangles), and a much lower ubiquinone reductase activity was observed. This suggests that monomeric DsbC mutants are a superior substrate to wild-type DsbC. Insert: to visualize the redox state of DsbC after incubation with DsbB and ubiquinone. Lane 1, purified oxidized DsbC; lanes 2–6, samples taken after 0.5, 1, 2, 3 and 4 h and precipitated with 5% TCA. Free thiols were modified with AMS essentially as described before (Kobayashi et al., 1997).
Similar articles
- Mutants in DsbB that appear to redirect oxidation through the disulfide isomerization pathway.
Pan JL, Sliskovic I, Bardwell JC. Pan JL, et al. J Mol Biol. 2008 Apr 11;377(5):1433-42. doi: 10.1016/j.jmb.2008.01.058. Epub 2008 Jan 31. J Mol Biol. 2008. PMID: 18325532 Free PMC article. - The disulphide isomerase DsbC cooperates with the oxidase DsbA in a DsbD-independent manner.
Vertommen D, Depuydt M, Pan J, Leverrier P, Knoops L, Szikora JP, Messens J, Bardwell JC, Collet JF. Vertommen D, et al. Mol Microbiol. 2008 Jan;67(2):336-49. doi: 10.1111/j.1365-2958.2007.06030.x. Epub 2007 Nov 25. Mol Microbiol. 2008. PMID: 18036138 Free PMC article. - Engineered DsbC chimeras catalyze both protein oxidation and disulfide-bond isomerization in Escherichia coli: Reconciling two competing pathways.
Segatori L, Paukstelis PJ, Gilbert HF, Georgiou G. Segatori L, et al. Proc Natl Acad Sci U S A. 2004 Jul 6;101(27):10018-23. doi: 10.1073/pnas.0403003101. Epub 2004 Jun 25. Proc Natl Acad Sci U S A. 2004. PMID: 15220477 Free PMC article. - Protein Disulfide Exchange by the Intramembrane Enzymes DsbB, DsbD, and CcdA.
Bushweller JH. Bushweller JH. J Mol Biol. 2020 Aug 21;432(18):5091-5103. doi: 10.1016/j.jmb.2020.04.008. Epub 2020 Apr 16. J Mol Biol. 2020. PMID: 32305461 Free PMC article. Review.
Cited by
- Legionella pneumophila utilizes a single-player disulfide-bond oxidoreductase system to manage disulfide bond formation and isomerization.
Kpadeh ZZ, Day SR, Mills BW, Hoffman PS. Kpadeh ZZ, et al. Mol Microbiol. 2015 Mar;95(6):1054-69. doi: 10.1111/mmi.12914. Epub 2015 Jan 30. Mol Microbiol. 2015. PMID: 25534767 Free PMC article. - Disulfide bond oxidoreductase DsbA2 of Legionella pneumophila exhibits protein disulfide isomerase activity.
Kpadeh ZZ, Jameson-Lee M, Yeh AJ, Chertihin O, Shumilin IA, Dey R, Day SR, Hoffman PS. Kpadeh ZZ, et al. J Bacteriol. 2013 Apr;195(8):1825-33. doi: 10.1128/JB.01949-12. Epub 2013 Feb 22. J Bacteriol. 2013. PMID: 23435972 Free PMC article. - The origami of thioredoxin-like folds.
Pan JL, Bardwell JC. Pan JL, et al. Protein Sci. 2006 Oct;15(10):2217-27. doi: 10.1110/ps.062268106. Protein Sci. 2006. PMID: 17008712 Free PMC article. - The conformational stability and biophysical properties of the eukaryotic thioredoxins of Pisum sativum are not family-conserved.
Aguado-Llera D, Martínez-Gómez AI, Prieto J, Marenchino M, Traverso JA, Gómez J, Chueca A, Neira JL. Aguado-Llera D, et al. PLoS One. 2011 Feb 22;6(2):e17068. doi: 10.1371/journal.pone.0017068. PLoS One. 2011. PMID: 21364950 Free PMC article. - FipB, an essential virulence factor of Francisella tularensis subsp. tularensis, has dual roles in disulfide bond formation.
Qin A, Zhang Y, Clark ME, Rabideau MM, Millan Barea LR, Mann BJ. Qin A, et al. J Bacteriol. 2014 Oct;196(20):3571-81. doi: 10.1128/JB.01359-13. Epub 2014 Aug 4. J Bacteriol. 2014. PMID: 25092026 Free PMC article.
References
- Bader M., Muse,W., Ballou,D.P., Gassner,C. and Bardwell,J.C. (1999) Oxidative protein folding is driven by the electron transport system. Cell, 98, 217–227. - PubMed
- Bader M.W., Xie,T., Yu,C.A. and Bardwell,J.C. (2000) Disulfide bonds are generated by quinone reduction. J. Biol. Chem., 275, 26082–26088. - PubMed
- Bardwell J.C., McGovern,K. and Beckwith,J. (1991) Identification of a protein required for disulfide bond formation in vivo. Cell, 67, 581–589. - PubMed
- Bessette P.H., Cotto,J.J., Gilbert,H.F. and Georgiou,G. (1999) In vivo and in vitro function of the Escherichia coli periplasmic cysteine oxidoreductase DsbG. J. Biol. Chem., 274, 7784–7792. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases