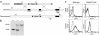B7-dependent T-cell costimulation in mice lacking CD28 and CTLA4 - PubMed (original) (raw)
B7-dependent T-cell costimulation in mice lacking CD28 and CTLA4
D A Mandelbrot et al. J Clin Invest. 2001 Apr.
Abstract
To examine whether B7 costimulation can be mediated by a molecule on T cells that is neither CD28 nor CTLA4, we generated mice lacking both of these receptors. CD28/CTLA4(-/-) mice resemble CD28(-/-) mice in having decreased expression of T-cell activation markers in vivo and decreased T-cell proliferation in vitro, as compared with wild-type mice. Using multiple approaches, we find B7-dependent costimulation in CD28/CTLA4(-/-) mice. The proliferation of CD28/CTLA4(-/-) T cells is inhibited by CTLA4-Ig and by the use of antigen-presenting cells lacking both B7-1 and B7-2. CD28/CTLA4(-/-) T-cell proliferation is increased by exposure to Chinese hamster ovary cells transfected with B7-1 or B7-2. Finally, administration of CTLA4-Ig to CD28/CTLA4(-/-) cardiac allograft recipients significantly prolongs graft survival. These data support the existence of an additional receptor for B7 molecules that is neither CD28 nor CTLA4.
Figures
Figure 1
Generation of mice lacking both CD28 and CTLA4. (a) Structure of the CD28 targeting construct. The hygromycin-resistance gene is flanked by small parts of exon 2 and 3. The thymidine kinase gene (TK) lies external to the 5′ genomic fragment. The intron-exon organization of the genomic clone is shown. Exons coding for the extracellular, transmembrane, and intracellular domain of CD28 are depicted as filled rectangles. The 0.9-kb cDNA probe used for Southern blot analysis is indicated as a thin bar and is located in exon 4, external to the genomic DNA fragment used in the targeting construct. Gene targeting in ES cells by homologous recombination results in the replacement of part of exon 2, the intron between exon 2 and 3, and part of exon 3 by the hygromycin gene. Southern blot analysis uses the acquisition of an additional _Eco_RV site in the hygromycin gene (shown in bold) to distinguish genomic and targeted DNA. (b) Southern blot analysis of the CD28 locus. Tail DNA from wild-type (+/+), heterozygotes (+/–), and CD28/CTLA4 double-knockout (–/–) littermates were digested with _Eco_RV and hybridized with the probe indicated in Figure 1a, demonstrating the two bands indicative of the endogenous (12 kb) or targeted (5 kb) configuration. (c) Analysis of CD28 and CTLA4 expression. Cell-surface staining of CD28 is assayed on naive CD4 T cells; CTLA4 is stained intracellularly in activated CD4 cells. Thick lines depict staining with anti-CD28 or anti–CTLA4; thin lines depict staining with the isotype-matched hamster IgG control Ab.
Figure 2
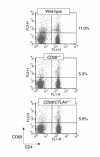
T-cell activation in naive mice. Freshly isolated lymph node cells from the indicated strains were stained for CD4 and CD69 and analyzed by flow cytometry. The percentages to the right of the dot plots are the proportion of CD4+ cells that are CD69+. This experiment is representative of five mice of each strain.
Figure 3
Proliferation of CD4+ T cells from wild-type (WT), CD28–/–, and CD28/CTLA4–/– mice in the presence of wild-type APCs. (a) Primary stimulation of CD4+ T cells with the indicated dilutions of anti-CD3. Proliferation on day 3 is shown. Data are representative of five experiments. (b) Secondary stimulation of CD4+ T cells with the indicated dilutions of anti-CD3. Proliferation on day 2 is shown. Data are representative of three experiments.
Figure 4
Cytokine production on day 2 after secondary stimulation of wild-type and CD28/CTLA4–/– CD4+ T cells with the indicated dilutions of anti-CD3. Data are representative of four experiments.
Figure 5
Proliferation of CD4+ T cells from wild-type, CD28–/–, and CD28/CTLA4–/– mice in the presence of syngeneic APCs. T cells from the indicated strains were stimulated in the presence of wild-type APCs with or without CTLA4-Ig or in the presence of B7-deficient APCs. Proliferation on day 3 is shown. Data are representative of five experiments.
Figure 6
Proliferation of CD4+ T cells from wild-type, CD28–/–, and CD28/CTLA4–/– mice in the presence of the indicated concentration of anti-CD3 and CHO cells transfected with I-Ad or I-Ad plus the indicated B7 molecules. Proliferation on day 3 is shown. Data are representative of three experiments.
Figure 7
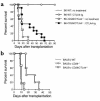
Cardiac transplantation studies. (a) BALB/c (H-2d) hearts were transplanted to B6 (H-2b) wild-type or CD28/CTLA4–/– recipients with or without CTLA4-Ig treatment. Graft survival was slightly prolonged in CD28/CTLA4–/– recipients (MST 15.5 days, n = 10), but there was no statistical significance compared with wild-type recipients (MST 9.4 days, n = 5; P = NS). CTLA4-Ig treatment in wild-type recipients prolonged graft survival significantly (MST > 90 days, n = 3; P < 0.02) compared with nontreated wild-type recipients. CTLA4-Ig treatment also prolonged graft survival in CD28/CTLA4–/– recipients significantly (MST 31.5 days, n = 11; P < 0.05) compared with nontreated CD28/CTLA4–/– recipients; however, that prolongation was much less than in wild-type recipients (P < 0.005). (b) Vascularized B6 (H-2b) hearts were transplanted to BALB/c (H-2d) wild-type, CD28–/–, or CD28/CTLA4–/– recipients. The graft survival in CD28–/– recipients was prolonged (MST 22.6 days, n = 5) compared with both wild-type (MST 9.0 days, n = 7; P < 0.001) and CD28/CTLA4–/– recipients (MST 10.3 days, n = 6; P < 0.002). CD28/CTLA4–/– recipients rejected B6 heart grafts at the same tempo as wild-type recipients.
Similar articles
- CD28-B7-mediated T cell costimulation in chronic cardiac allograft rejection: differential role of B7-1 in initiation versus progression of graft arteriosclerosis.
Kim KS, Denton MD, Chandraker A, Knoflach A, Milord R, Waaga AM, Turka LA, Russell ME, Peach R, Sayegh MH. Kim KS, et al. Am J Pathol. 2001 Mar;158(3):977-86. doi: 10.1016/S0002-9440(10)64044-8. Am J Pathol. 2001. PMID: 11238045 Free PMC article. - Cytotoxic T lymphocyte antigen 4 (CTLA4) blockade accelerates the acute rejection of cardiac allografts in CD28-deficient mice: CTLA4 can function independently of CD28.
Lin H, Rathmell JC, Gray GS, Thompson CB, Leiden JM, Alegre ML. Lin H, et al. J Exp Med. 1998 Jul 6;188(1):199-204. doi: 10.1084/jem.188.1.199. J Exp Med. 1998. PMID: 9653096 Free PMC article. - CD28/CTLA-4 and CD80/CD86 families: signaling and function.
Slavik JM, Hutchcroft JE, Bierer BE. Slavik JM, et al. Immunol Res. 1999;19(1):1-24. doi: 10.1007/BF02786473. Immunol Res. 1999. PMID: 10374692 Review. - Expression of B7 molecules in recipient, not donor, mice determines the survival of cardiac allografts.
Mandelbrot DA, Furukawa Y, McAdam AJ, Alexander SI, Libby P, Mitchell RN, Sharpe AH. Mandelbrot DA, et al. J Immunol. 1999 Oct 1;163(7):3753-7. J Immunol. 1999. PMID: 10490971 - Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation.
Salomon B, Bluestone JA. Salomon B, et al. Annu Rev Immunol. 2001;19:225-52. doi: 10.1146/annurev.immunol.19.1.225. Annu Rev Immunol. 2001. PMID: 11244036 Review.
Cited by
- Activation and inhibition of lymphocytes by costimulation.
Frauwirth KA, Thompson CB. Frauwirth KA, et al. J Clin Invest. 2002 Feb;109(3):295-9. doi: 10.1172/JCI14941. J Clin Invest. 2002. PMID: 11827987 Free PMC article. Review. No abstract available. - Loss of myeloid related protein-8/14 exacerbates cardiac allograft rejection.
Shimizu K, Libby P, Rocha VZ, Folco EJ, Shubiki R, Grabie N, Jang S, Lichtman AH, Shimizu A, Hogg N, Simon DI, Mitchell RN, Croce K. Shimizu K, et al. Circulation. 2011 Dec 20;124(25):2920-32. doi: 10.1161/CIRCULATIONAHA.110.009910. Epub 2011 Dec 5. Circulation. 2011. PMID: 22144572 Free PMC article. - CD28 costimulation is required for the expression of T-cell-dependent cell-mediated immunity against blood-stage Plasmodium chabaudi malaria parasites.
Rummel T, Batchelder J, Flaherty P, LaFleur G, Nanavati P, Burns JM, Weidanz WP. Rummel T, et al. Infect Immun. 2004 Oct;72(10):5768-74. doi: 10.1128/IAI.72.10.5768-5774.2004. Infect Immun. 2004. PMID: 15385476 Free PMC article. - Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses.
Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Butte MJ, et al. Immunity. 2007 Jul;27(1):111-22. doi: 10.1016/j.immuni.2007.05.016. Epub 2007 Jul 12. Immunity. 2007. PMID: 17629517 Free PMC article. - Cytotoxic T-Lymphocyte-Associated Protein 4 Haploinsufficiency-Associated Inflammation Can Occur Independently of T-Cell Hyperproliferation.
Le Coz C, Nolan BE, Trofa M, Kamsheh AM, Khokha MK, Lakhani SA, Novelli A, Zackai EH, Sullivan KE, Briuglia S, Bhatti TR, Romberg N. Le Coz C, et al. Front Immunol. 2018 Jul 24;9:1715. doi: 10.3389/fimmu.2018.01715. eCollection 2018. Front Immunol. 2018. PMID: 30087679 Free PMC article.
References
- McAdam AJ, Schweitzer AN, Sharpe AH. The role of B7 co-stimulation in activation and differentiation of CD4+ and CD8+ T cells. Immunol Rev. 1998;165:231–247. - PubMed
- Oosterwegel MA, Greenwald RJ, Mandelbrot DA, Lorsbach RB, Sharpe AH. CTLA-4 and T cell activation. Curr Opin Immunol. 1999;11:294–300. - PubMed
- Mandelbrot DA, et al. Expression of B7 molecules in recipient, not donor, mice determines the survival of cardiac allografts. J Immunol. 1999;163:3753–3757. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI-34965/AI/NIAID NIH HHS/United States
- AI-38310/AI/NIAID NIH HHS/United States
- AI-41584/AI/NIAID NIH HHS/United States
- AI-39671/AI/NIAID NIH HHS/United States
- R37 AI038310/AI/NIAID NIH HHS/United States
- P01 AI041521/AI/NIAID NIH HHS/United States
- P01 AI039671/AI/NIAID NIH HHS/United States
- CA-75174/CA/NCI NIH HHS/United States
- AI-41521/AI/NIAID NIH HHS/United States
- R01 AI038310/AI/NIAID NIH HHS/United States
- R01 AI034965/AI/NIAID NIH HHS/United States
- HL 43364/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases