IL-1-induced NFkappa B and c-Jun N-terminal kinase (JNK) activation diverge at IL-1 receptor-associated kinase (IRAK) - PubMed (original) (raw)
IL-1-induced NFkappa B and c-Jun N-terminal kinase (JNK) activation diverge at IL-1 receptor-associated kinase (IRAK)
X Li et al. Proc Natl Acad Sci U S A. 2001.
Abstract
Mutant I1A cells, lacking IL-1 receptor-associated kinase (IRAK) mRNA and protein, have been used to study the involvement of IRAK in NFkappaB and c-Jun N-terminal kinase (JNK) activation. A series of IRAK deletion constructs were expressed in I1A cells, which were then tested for their ability to respond to IL-1. Both the N-terminal death domain and the C-terminal region of IRAK are required for IL-1-induced NFkappaB and JNK activation, whereas the N-proximal undetermined domain is required for the activation of NFkappaB but not JNK. The phosphorylation and ubiquitination of IRAK deletion mutants correlate tightly with their ability to activate NFkappaB in response to IL-1, but IRAK can mediate IL-1-induced JNK activation without being phosphorylated. These studies reveal that the IL-1-induced signaling pathways leading to NFkappaB and JNK activation diverge either at IRAK or at a point nearer to the receptor.
Figures
Figure 1
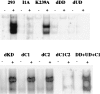
Deletion analysis of IRAK. Constructs encoding the point mutant K239A and the deletion mutants were transiently cotransfected into IRAK-null I1A cells with E-selectin-Luc. The data are presented as fold induction of luciferase activity in IL-1-treated cells compared with the induction in untreated cells. The averages and standard deviations from four independent experiments are shown. DD, death domain; UD, domain of unknown function; KD, kinase domain; dDD, deletion of death domain; dUD, deletion of undetermined domain; dKD, deletion of kinase domain; dC_n_, deletion of C-terminal domain n; C_n_, C-terminal domain n.
Figure 2
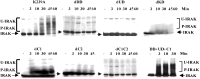
Domains of IRAK required for IL-1-induced NFκB activation. IRAK-null I1A cells stably transfected with K239A or the deletion mutants were either untreated (−) or treated with 100 units/ml IL-1 for 15 min (+). Whole-cell extracts were used for gel-shift assays. Wild-type 293 cells (WT) and IRAK-null I1A cells (I1A) were used as positive and negative controls, respectively.
Figure 3
Domains of IRAK required for IL-1-induced JNK activation. IRAK-null I1A cells stably transfected with IRAK deletion mutants were either untreated (−) or treated with 100 units/ml IL-1 (I) or with 40 J/m2 UV radiation (UV). Cell extracts were analyzed with anti-phospho-JNK after Western transfer. Wild-type 293 cells (WT) and IRAK-null I1A cells (I1A) were used as positive and negative controls, respectively. Data from one of four experiments are shown.
Figure 4
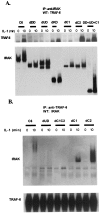
IL-1-induced IRAK modification and degradation. IRAK-null I1A cells transfected with K239A or the deletion mutants were either untreated (−) or treated with IL-1 for the number of minutes indicated. Cell extracts were analyzed with anti-IRAK by the Western procedure. IRAK, unmodified IRAK (arrow); P-IRAK, phosphorylated IRAK; U-IRAK, ubiquitinated IRAK.
Figure 5
IL-1-induced phosphorylation of IRAK. Wild-type (WT) 293 cells or IRAK-null I1A cells transfected with dUD were incubated in phosphate-free medium containing [32P]orthophosphate for 4 h and then either left untreated (−) or treated with 100 units/ml IL-1 for 15 min (+). Cell extracts were then immunoprecipitated with anti-IRAK and separated by SDS/(7%)PAGE. (A) Analysis of phosphorylated IRAK (P-IRAK) by autoradiography. (B) Western analysis of IRAK.
Figure 6
Interaction of IRAK with TRAF6. IRAK-null I1A cells transfected with IRAK deletion mutants were either untreated (−) or treated with 100 units/ml IL-1 for 15 min (+). (A) Extracts from cells transfected with dDD, dUD, dKD, dC1, and dC2; DD + UD + C1 cells were immunoprecipitated with anti-IRAK and probed with anti-TRAF6 and anti-IRAK. (B) Anti-IRAK antibody (Santa Cruz Biotechnology; recognizes 440–712 residues of IRAK) does not immunoprecipitate dC1C2 well, although it does recognize dC1C2 on a Western blot (Fig. 4). Therefore, dC1C2 and a few other IRAK deletion mutants (dUD, dC1, and dC2) were immunoprecipitated with anti-TRAF6 and probed with anti-IRAK and anti-TRAF6. Wild-type 293 cells (WT) were used as a positive control.
References
- Dinarello C A. Blood. 1996;87:2095–2147. - PubMed
- Barnes P J, Karin M. N Engl J Med. 1997;336:1066–1071. - PubMed
- O'Neill L A J. Biochim Biophys Acta. 1995;1266:31–44. - PubMed
- O'Neill L A J. Biochem Soc Trans. 1997;25:295–302. - PubMed
- Greenfeder S A, Nunes P, Kwee L, Labow M, Chizzonite R A, Ju G. J Biol Chem. 1995;270:13757–13765. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous