Expression of the telomerase catalytic subunit, hTERT, induces resistance to transforming growth factor beta growth inhibition in p16INK4A(-) human mammary epithelial cells - PubMed (original) (raw)
Expression of the telomerase catalytic subunit, hTERT, induces resistance to transforming growth factor beta growth inhibition in p16INK4A(-) human mammary epithelial cells
M R Stampfer et al. Proc Natl Acad Sci U S A. 2001.
Abstract
Failures to arrest growth in response to senescence or transforming growth factor beta (TGF-beta) are key derangements associated with carcinoma progression. We report that activation of telomerase activity may overcome both inhibitory pathways. Ectopic expression of the human telomerase catalytic subunit, hTERT, in cultured human mammary epithelial cells (HMEC) lacking both telomerase activity and p16(INK4A) resulted in gaining the ability to maintain indefinite growth in the absence and presence of TGF-beta. The ability to maintain growth in TGF-beta was independent of telomere length and required catalytically active telomerase capable of telomere maintenance in vivo. The capacity of ectopic hTERT to induce TGF-beta resistance may explain our previously described gain of TGF-beta resistance after reactivation of endogenous telomerase activity in rare carcinogen-treated HMEC. In those HMEC that overcame senescence, both telomerase activity and TGF-beta resistance were acquired gradually during a process we have termed conversion. This effect of hTERT may model a key change occurring during in vivo human breast carcinogenesis.
Figures
Figure 1
Schematic chart of hTERT transduction of (A) postselection p16(−) 184 HMEC at passages 11 and 18; (B) conditional immortal 184A1 (preconversion at passage 12, early conversion at passage 22); (C) preselection p16(+) 184 HMEC at passage 3.
Figure 2
Growth of postselection HMEC ± hTERT and ± TGF-β. 184 HMEC infected at passage 11 with either hTERT-containing [hTERT-184(11p)] or control retroviruses [LXSN-184(11p)] and uninfected 184 HMEC were assayed for growth ± TGF-β at the indicated passages.
Figure 3
hTERT expression induces TGF-β resistance in (A) postselection and (B) conditionally immortal HMEC. Data from Tables 1 and 2 and from ref. and unpublished work for the p53(+) line 184A1 and the p53(−/−) line 184AA3 are plotted to illustrate the rapid gain of TGF-β resistance in postselection HMEC vs. the gradual gain of resistance, with similar kinetics after telomerase activation, in p53(+) and (−/−) immortal HMEC lines and in hTERT-transduced 184A1. Closed arrows indicate passage of infection; open arrows indicate passage when telomerase activity was first detected in the uninfected lines.
Figure 4
Ectopic hTERT causes telomeres to lengthen in normal and conditionally immortal HMEC, whereas telomeres become critically short in immortal HMEC undergoing conversion. (A) Postselection 184 HMEC; hTERT-184(11p) shows rapid telomere lengthening whereas LXSN-184(11p) telomeres continue to shorten to a mean TRF of ≈5 kb at senescence. Both (B) conditional immortal 184A1 transduced at passage 12 and (C) conditional immortal 184A1 transduced at passage 22 with hTERT show rapid telomere lengthening, whereas LSXN-184A1 undergoing conversion show continued telomere erosion to faint critically short mean TRF of ≈2 kb, followed by stabilization.
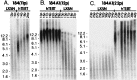
Figure 5
Rare hTERT immortalization of preselection 184 HMEC is associated with gradual down-regulation of p16 and acquisition of TGF-β resistance. (A) Decreasing p16 expression (brown precipitate) in individual cells with increasing passage in the rare preselection HMEC that gained immortality after hTERT transduction at passage 3 [hTERT-184(3p)]. p16(−) postselection HMEC transduced with hTERT [184-TERT(11p)] are shown for comparison. (Bar = 100 μm.) (B) Western blot analysis showing decreasing p16 expression with increasing passage in hTERT-184(3p). (C) Increasing ability to grow in TGF-β correlates with decreasing p16 expression in hTERT-184(3p). Growth was assayed as described in_Methods_; LI was determined from five separate fields at the indicated days postseeding.
Similar articles
- Molecular changes accompanying senescence and immortalization of cultured human mammary epithelial cells.
Yaswen P, Stampfer MR. Yaswen P, et al. Int J Biochem Cell Biol. 2002 Nov;34(11):1382-94. doi: 10.1016/s1357-2725(02)00047-x. Int J Biochem Cell Biol. 2002. PMID: 12200033 Review. - Events in the immortalizing process of primary human mammary epithelial cells by the catalytic subunit of human telomerase.
Kim H, Farris J, Christman SA, Kong BW, Foster LK, O'Grady SM, Foster DN. Kim H, et al. Biochem J. 2002 Aug 1;365(Pt 3):765-72. doi: 10.1042/BJ20011848. Biochem J. 2002. PMID: 11978176 Free PMC article. - Human keratinocytes that express hTERT and also bypass a p16(INK4a)-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics.
Dickson MA, Hahn WC, Ino Y, Ronfard V, Wu JY, Weinberg RA, Louis DN, Li FP, Rheinwald JG. Dickson MA, et al. Mol Cell Biol. 2000 Feb;20(4):1436-47. doi: 10.1128/MCB.20.4.1436-1447.2000. Mol Cell Biol. 2000. PMID: 10648628 Free PMC article. - Raf-1-induced growth arrest in human mammary epithelial cells is p16-independent and is overcome in immortal cells during conversion.
Olsen CL, Gardie B, Yaswen P, Stampfer MR. Olsen CL, et al. Oncogene. 2002 Sep 12;21(41):6328-39. doi: 10.1038/sj.onc.1205780. Oncogene. 2002. PMID: 12214273 - Soothing the watchman: telomerase reduces the p53-dependent cellular stress response.
Beliveau A, Yaswen P. Beliveau A, et al. Cell Cycle. 2007 Jun 1;6(11):1284-7. doi: 10.4161/cc.6.11.4298. Epub 2007 Jun 13. Cell Cycle. 2007. PMID: 17534147 Review.
Cited by
- Telomerase in Cancer: Function, Regulation, and Clinical Translation.
Robinson NJ, Schiemann WP. Robinson NJ, et al. Cancers (Basel). 2022 Feb 5;14(3):808. doi: 10.3390/cancers14030808. Cancers (Basel). 2022. PMID: 35159075 Free PMC article. Review. - Cellular aging beyond cellular senescence: Markers of senescence prior to cell cycle arrest in vitro and in vivo.
Ogrodnik M. Ogrodnik M. Aging Cell. 2021 Apr;20(4):e13338. doi: 10.1111/acel.13338. Epub 2021 Mar 12. Aging Cell. 2021. PMID: 33711211 Free PMC article. Review. - Superresolution microscopy reveals linkages between ribosomal DNA on heterologous chromosomes.
Potapova TA, Unruh JR, Yu Z, Rancati G, Li H, Stampfer MR, Gerton JL. Potapova TA, et al. J Cell Biol. 2019 Aug 5;218(8):2492-2513. doi: 10.1083/jcb.201810166. Epub 2019 Jul 3. J Cell Biol. 2019. PMID: 31270138 Free PMC article. - The non-canonical functions of telomerase: to turn off or not to turn off.
Romaniuk A, Paszel-Jaworska A, Totoń E, Lisiak N, Hołysz H, Królak A, Grodecka-Gazdecka S, Rubiś B. Romaniuk A, et al. Mol Biol Rep. 2019 Feb;46(1):1401-1411. doi: 10.1007/s11033-018-4496-x. Epub 2018 Nov 17. Mol Biol Rep. 2019. PMID: 30448892 Review. - Different culture media modulate growth, heterogeneity, and senescence in human mammary epithelial cell cultures.
Lee JK, Bloom J, Zubeldia-Plazaola A, Garbe JC, Stampfer MR, LaBarge MA. Lee JK, et al. PLoS One. 2018 Oct 1;13(10):e0204645. doi: 10.1371/journal.pone.0204645. eCollection 2018. PLoS One. 2018. PMID: 30273377 Free PMC article.
References
- Kim N W, Piatyszek M A, Prowse K R, Harley C B, West M D, Ho P L C, Coviello G M, Wright W E, Weinrich S L, Shay J W. Science. 1994;266:2011–2015. - PubMed
- Fynan T M, Reiss M. Crit Rev Oncog. 1993;4:493–540. - PubMed
- Bodnar A G, Ouellette M, Frolkis M, Holt S E, Chiu C-P, Morin G B, Harley C B, Shay J W, Lichtsteiner S, Wright W E. Science. 1998;279:349–352. - PubMed
- Morales C P, Holt S E, Ouellette M, Kaur J, Yan Y, Wilson K S, White M A, Wright W E, Shay J W. Nat Gen. 1999;21:115–118. - PubMed
- Jiang W-R, Jimenez G, Chang E, Frolkis M, Kusler B, Sage J, Beeche M, Bodnar A G, Wahl G M, Tlsty T D, Chiu C-P. Nat Gen. 1999;21:111–114. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources