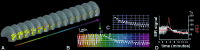Mechanisms of migraine aura revealed by functional MRI in human visual cortex - PubMed (original) (raw)
. 2001 Apr 10;98(8):4687-92.
doi: 10.1073/pnas.071582498. Epub 2001 Apr 3.
M Sanchez Del Rio, O Wu, D Schwartz, D Bakker, B Fischl, K K Kwong, F M Cutrer, B R Rosen, R B Tootell, A G Sorensen, M A Moskowitz
Affiliations
- PMID: 11287655
- PMCID: PMC31895
- DOI: 10.1073/pnas.071582498
Mechanisms of migraine aura revealed by functional MRI in human visual cortex
N Hadjikhani et al. Proc Natl Acad Sci U S A. 2001.
Abstract
Cortical spreading depression (CSD) has been suggested to underlie migraine visual aura. However, it has been challenging to test this hypothesis in human cerebral cortex. Using high-field functional MRI with near-continuous recording during visual aura in three subjects, we observed blood oxygenation level-dependent (BOLD) signal changes that demonstrated at least eight characteristics of CSD, time-locked to percept/onset of the aura. Initially, a focal increase in BOLD signal (possibly reflecting vasodilation), developed within extrastriate cortex (area V3A). This BOLD change progressed contiguously and slowly (3.5 +/- 1.1 mm/min) over occipital cortex, congruent with the retinotopy of the visual percept. Following the same retinotopic progression, the BOLD signal then diminished (possibly reflecting vasoconstriction after the initial vasodilation), as did the BOLD response to visual activation. During periods with no visual stimulation, but while the subject was experiencing scintillations, BOLD signal followed the retinotopic progression of the visual percept. These data strongly suggest that an electrophysiological event such as CSD generates the aura in human visual cortex.
Figures
Figure 1
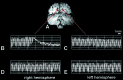
Ictal and interictal BOLD responses in human visual cortex. A representative functional MRI slice is shown (A). The slice plane was oriented near-perpendicular to the calcarine fissure, so that cerebellum occupies the lower portion of the figure, and occipital lobe occupies the upper portion. (B_–_E) Representative BOLD responses over time, taken from single voxels within homologous areas of the occipital lobe (B and D, Right vs.C and E, Left), as designated by green arrows. Time is shown on the x axis, and levels of MR modulation are shown on the y axis. The stimulus-driven signal oscillation in_B_–E is the BOLD responses to 16-s presentations of the checkerboard visual stimulus (on response), relative to the intervening 16-s presentations of a black screen with a fixation point (off response). (D and E) Normal BOLD modulation during an interictal period for each hemisphere. (B and C) The BOLD responses during a migraine aura affecting only the right hemisphere (B) (see Fig. 2). Perturbations did not appear in the left hemisphere during the ictal (C), or interictal scans (D and E).
Figure 2
Time-dependent BOLD activity changes from a single region of interest in VI, acquired before and during episodes of either spontaneous (C) or induced (B) visual aura. (A) A series of anatomical images, including BOLD activity on “inflated” cortical hemispheres showing the medial bank (similar to a conventional midsagittal view). Images were sampled at 32-s intervals, showing the same region of interest (circles) in V1. (B) The MR signal perturbation over time from the circled region of interest; the perturbation is similar to that in Fig. 1_B_. Variations in time are color-coded (deep red to magenta), and the four colored circles match corresponding times within the V1 region of interest. The slice prescription failed to include a few mm in the most posterior part of the occipital pole in that induced attack, so activation is not revealed in any of these images.B shows that before the onset of the aura, the BOLD response to visual stimulation shows a normal, oscillating activation pattern. After the onset of aura (green arrow), the BOLD response showed a marked increase in mean level (α), a marked suppression to light modulation (β), followed by a partial recovery of the response to light modulation at decreased mean level (γ; −3% to −6%). (C) Data from a spontaneous attack (subject M.C.), captured ≈18 min after the onset of the visual symptoms affecting the right hemifield. The data represent the time course in left visual area V1, at an eccentricity of ≈20° of visual angle. (D) A superimposition of CBF changes seen in the rat during CSD (as described by Lauritzen et al. in ref. 46) with the MR signal data shown in A. Note that the timing of the hyperemia (3–4.5 min in CSD vs. 3.3 ± 1.9 min in migraine aura) is remarkably similar in these two quite different data sets. The amplitude of the hyperemia is different in the two conditions, presumably because of differences in the blood flow measurement techniques used (laser doppler versus BOLD) and the nonlinear relationship between blood flow and BOLD signal.
Figure 3
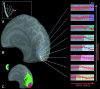
Spreading suppression of cortical activation during migraine aura. (A) A drawing showing the progression over 20 min of the scintillations and the visual field defect affecting the left hemifield, as described by the patient (P.R.). The fixation point appears as a small white cross. The red line shows the overall direction of progression of the visual percept. The front of the scintillation at different times within the aura is indicated by a white line. (B) A reconstruction of the same patient's brain (P.R.), based on anatomical MR data. The posterior medial aspect of occipital lobe is shown in an inflated cortex format. In this format, the cortical sulci and gyri appear in darker and lighter gray, respectively, on a computationally inflated surface. MR signal changes over time are shown to the right. Each time course was recorded from one in a sequence of voxels that were sampled along the calcarine sulcus, in the primary visual cortex (V1), from the posterior pole to more anterior location, as indicated by arrowheads. A similar BOLD response was found within all of the extrastriate areas, differing only in the time of onset of the MR perturbation The MR perturbations developed earlier in the foveal representation, compared with more eccentric representations of retinotopic visual cortex. This finding was consistent with the progression of the aura from central to peripheral eccentricities in the corresponding visual field (A and C). (C) The MR maps of retinotopic eccentricity from this same subject, acquired during interictal scans. As shown in the logo in the upper left, voxels that show retinotopically specific activation in the fovea are coded in red (centered at 1.5° eccentricity). Parafoveal eccentricities are shown in blue, and more peripheral eccentricities are shown in green (centered at 3.8° and 10.3°, respectively).
Figure 4
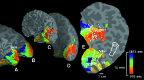
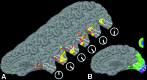
Source localization and time of onset of the MR signal perturbations. (A and C) The data on (normally) folded right hemispheric cortex; (B and D) the same data on inflated cortical surface (as in Figs. 2 and 3). (E) A fully flattened view of the cortical surface, as shown in previous publications (–26, 37, 53). (A and_B_) A view of the exposed medial bank from the posterior pole. (C and D) Shown is the entire hemisphere, from a posterior-medial view. Pos indicates the parieto-occipital sulcus. As described in Fig. 2, activation data were not acquired from the extreme posterior tip of the occipital pole. Cortical locations showing the first BOLD perturbations are coded in red (E). Locations showing the BOLD perturbations at progressively later times are coded by green and blue (see pseudocolor scale to the right). The aura-related changes appeared first in extrastriate cortex (V3A, closely followed by V3 and V2), then progressed into V1. The spread of the aura began, and was most systematic, in the representation of the lower visual field (upper bank), becoming less regular as it progressed into the representation of the upper visual field.
Figure 5
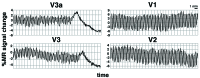
Time-course evidence for a migraine origin in visual area V3A, taken from the same subject (P.R.) illustrated in Fig. 6, but in a different migraine attack. The slice prescription did include the most posterior part of the occipital pole. Each of the four panels shows the BOLD signals from a voxel in either area V3A, or V3, or V2 or V1. All voxels were sampled from approximately equivalent (parafoveal) retinotopic eccentricities. Time is represented along the x axis, and the range is equal and synchronized in each of the four panels. MR amplitude (indicated on the y axis) varied slightly in the different pixels and visual areas. Area V3A showed the first BOLD perturbation. Within a few minutes, the perturbation spread into adjacent area V3. The perturbations then spread further posteriorly into areas V2 and V1—but in those areas, the perturbations occurred at times following the time range shown.
Figure 6
Progression of the scintillations in the dark, without the flickering checkerboard stimuli. (A) A series of images of the brain of the subject at different times, from the beginning of the scanning session (see the little clocks below). The primary visual cortex lies within the white line. Initially, no activation can be seen in V1. However, with the subjective apparition of the scintillations (after 20 min, see clock), activation appears in V1, that progresses from the foveal representation of the visual field to more peripheral representations, paralleling the progression of the scintillations described by the subject. (B) A medial view of the subject's brain with the MR maps of retinotopic eccentricity acquired during interictal scans. (see above, Fig. 3).
Similar articles
- Heterogenous migraine aura symptoms correlate with visual cortex functional magnetic resonance imaging responses.
Arngrim N, Hougaard A, Ahmadi K, Vestergaard MB, Schytz HW, Amin FM, Larsson HBW, Olesen J, Hoffmann MB, Ashina M. Arngrim N, et al. Ann Neurol. 2017 Dec;82(6):925-939. doi: 10.1002/ana.25096. Epub 2017 Dec 4. Ann Neurol. 2017. PMID: 29130510 - Clinical correlates of mathematical modeling of cortical spreading depression: Single-cases study.
Kroos JM, de Tommaso M, Stramaglia S, Vecchio E, Burdi N, Gerardo-Giorda L. Kroos JM, et al. Brain Behav. 2019 Oct;9(10):e01387. doi: 10.1002/brb3.1387. Epub 2019 Sep 10. Brain Behav. 2019. PMID: 31503424 Free PMC article. - History of migraine with aura and cortical spreading depression from 1941 and onwards.
Tfelt-Hansen PC. Tfelt-Hansen PC. Cephalalgia. 2010 Jul;30(7):780-92. doi: 10.1111/j.1468-2982.2009.02015.x. Cephalalgia. 2010. PMID: 19740119 Review. - Vision and migraine.
Vincent MB. Vincent MB. Headache. 2015 Apr;55(4):595-9. doi: 10.1111/head.12531. Epub 2015 Mar 11. Headache. 2015. PMID: 25758366 Review.
Cited by
- Cortical Spreading Depression Causes Unique Dysregulation of Inflammatory Pathways in a Transgenic Mouse Model of Migraine.
Eising E, Shyti R, 't Hoen PAC, Vijfhuizen LS, Huisman SMH, Broos LAM, Mahfouz A, Reinders MJT, Ferrari MD, Tolner EA, de Vries B, van den Maagdenberg AMJM. Eising E, et al. Mol Neurobiol. 2017 May;54(4):2986-2996. doi: 10.1007/s12035-015-9681-5. Epub 2016 Mar 31. Mol Neurobiol. 2017. PMID: 27032388 Free PMC article. - Familial and sporadic hemiplegic migraine: diagnosis and treatment.
Pelzer N, Stam AH, Haan J, Ferrari MD, Terwindt GM. Pelzer N, et al. Curr Treat Options Neurol. 2013 Feb;15(1):13-27. doi: 10.1007/s11940-012-0208-3. Curr Treat Options Neurol. 2013. PMID: 23203776 - Alterations in metabolic flux in migraine and the translational relevance.
Grech O, Sassani M, Terwindt G, Lavery GG, Mollan SP, Sinclair AJ. Grech O, et al. J Headache Pain. 2022 Sep 30;23(1):127. doi: 10.1186/s10194-022-01494-w. J Headache Pain. 2022. PMID: 36175833 Free PMC article. Review. - The Revolution in Migraine Genetics: From Aching Channels Disorders to a Next-Generation Medicine.
Pellacani S, Sicca F, Di Lorenzo C, Grieco GS, Valvo G, Cereda C, Rubegni A, Santorelli FM. Pellacani S, et al. Front Cell Neurosci. 2016 Jun 13;10:156. doi: 10.3389/fncel.2016.00156. eCollection 2016. Front Cell Neurosci. 2016. PMID: 27378853 Free PMC article. Review. - Large-scale mass spectrometry imaging investigation of consequences of cortical spreading depression in a transgenic mouse model of migraine.
Carreira RJ, Shyti R, Balluff B, Abdelmoula WM, van Heiningen SH, van Zeijl RJ, Dijkstra J, Ferrari MD, Tolner EA, McDonnell LA, van den Maagdenberg AM. Carreira RJ, et al. J Am Soc Mass Spectrom. 2015 Jun;26(6):853-61. doi: 10.1007/s13361-015-1136-8. Epub 2015 Apr 16. J Am Soc Mass Spectrom. 2015. PMID: 25877011 Free PMC article.
References
- Russell M B, Olesen J. Brain. 1996;119:355–361. - PubMed
- Leao A A. J Neurophysiol. 1944;7:359–390.
- Leao A A. Funct Neurol. 1986;1:363–366. - PubMed
- Olesen J, Tfelt-Hansen P, Welch K M A. The Headaches. Philadelphia: Lippincott; 2000.
Publication types
MeSH terms
Grants and funding
- K08-NS01803A/NS/NINDS NIH HHS/United States
- P41 RR 14075/RR/NCRR NIH HHS/United States
- P41 RR014075/RR/NCRR NIH HHS/United States
- 5 PO1 NS 35611/NS/NINDS NIH HHS/United States
- EY 07980/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous