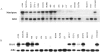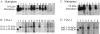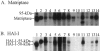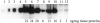Matriptase and HAI-1 are expressed by normal and malignant epithelial cells in vitro and in vivo - PubMed (original) (raw)
Matriptase and HAI-1 are expressed by normal and malignant epithelial cells in vitro and in vivo
M Oberst et al. Am J Pathol. 2001 Apr.
Abstract
Matriptase and its cognate, Kunitz-type serine protease inhibitor, HAI-1, comprise a newly characterized extracellular matrix-degrading protease system that may function as an epithelial membrane activator for other proteases and latent growth factors. Both enzyme and inhibitor have been detected in breast cancer cells, immortalized mammary epithelial cells, and human milk, but not in cultured fibroblasts nor in fibrosarcoma cells. To test the hypothesis that this system is expressed by normal breast epithelium, invasive breast cancers, and other cancers of an epithelial origin (carcinomas) but not in cancers of a mesenchymal origin, we have expanded our expression analysis of matriptase and HAI-1 in vitro and in vivo. Matriptase and HAI-1 were detected at the protein and mRNA levels both in hormone-dependent and hormone-independent cultured breast cancer cells, and this expression correlated with the expression of the epithelial markers E-cadherin or ZO-1. However, none of the breast cancer cell lines tested that express the mesenchymal marker vimentin express matriptase or HAI-1, consistent with an epithelial-selective expression of this system. Expression of matriptase, as determined by Western blot analysis, was observed in primary human breast, gynecological, and colon carcinomas, but not in stromal-derived ovarian tumors and human sarcomas of various origins and histological grades. The epithelial-selective expression of matriptase and HAI-1 was further confirmed in human breast cancers by immunohistochemistry and in situ hybridization, where the expression of the protease and the inhibitor were found in the carcinoma cells and in surrounding normal breast epithelia. The expression of the matriptase/HAI-1 system by malignant epithelial cells in vivo suggests a possible role for this protease in multiple aspects of the pathophysiology of epithelial malignancy, including invasion and metastasis.
Figures
Figure 1.
Expression analysis of matriptase and HAI-1 in immortalized human breast epithelial, human breast cancer, and ovarian cancer cell lines. Ten micrograms of total RNA were examined for each breast or ovarian cell line by Northern blot analysis using matriptase-specific (A) or HAI-1-specific (B) riboprobes. Analysis of cell lines included two immortalized breast epithelial lines (MCF-10A and A1N4), four ER+ breast cancer cell lines (MCF-7, ZR-75-1, T47D, BT474), nine ER− breast cancer cell lines (SKBR3, MDA-MB-468, -453, -436, -435, -157, and -231, BT549, and Hs578t), and three ovarian cancer cell lines (SKOV3, PA-1, and OVCAR-3). Matriptase expression always correlated with HAI-1 expression, and both were found in two of two immortalized breast epithelial cell lines, four of four ER+ breast cancer cell lines, three of nine ER− breast cancer cell lines, and in one of three ovarian cancer cell lines.
Figure 2.
Expression analysis of matriptase in mammary tissues. Samples of proteins were extracted using RIPA buffer from normal breast tissue surrounding the breast tumor of three different patients (lanes 13 to 15) and tumors of 10 different patients (lanes 3 to 12). Proteins (50 μg per lane) were separated by SDS-polyacrylamide gel electrophoresis, transferred to polyvinylidene fluoride membrane, and probed by anti-matriptase mAb 21-9 (A) and anti-HAI-1 mAb M19 (B). The positions of matriptase (70 kd), HAI-1 (55-kd membrane-bound form and 50-kd fragment), and the 95-kd matriptase/HAI-1 complex were indicated according to the samples from the cell-conditioned medium (lanes 1) and the membrane fractions (lanes 2) of T-47D breast cancer cells.
Figure 3.
Expression analysis of matriptase in gynecological tumors. A and B: Samples of proteins (50 μg per lane), which were extracted by RIPA buffer from nine ovarian carcinomas (lanes 3 to 11) and three stromal-derived tumors, including two fibrothecomas (lanes 12 and 13) and one granulosa cell tumor (lane 14), were analyzed by immunoblot using anti-matriptase mAb 21-9 and anti-HAI-1 mAb M19. The positions of matriptase, HAI-1 and the 95-kd matriptase/HAI-1 complex were indicated according to the samples from the cell-conditioned medium (lanes 1) and the membrane fractions (lanes 2) of T-47D cells. C and D: Samples of proteins from four uterine carcinomas (lanes 3, 5, 7, and 8) and two patient-matched normal tissues surrounding tumors (lanes 4 and 6) were probed by immunoblot using anti-matriptase mAb 21-9 and anti-HAI-1 mAb M19. The positions of matriptase, HAI-1, and the 95-kd matriptase/HAI-1 complex are indicated, as described above.
Figure 4.
Expression analysis of matriptase in human colon tumors. Protein samples (50 μg per lane) that were extracted by RIPA buffer from nine colon carcinomas (lanes 3 to 10) and four normal colon specimens (lanes 11 to 14) were examined by Western blot using anti-matriptase mAb 21-9 (A) and anti-HAI-1 mAb M19 (B). The positions of matriptase, HAI-1, and its 95-kd matriptase/HAI-1 complex, were indicated according to the samples from the cell-conditioned medium (lanes 1) and the membrane fractions (lanes 2) of T-47D cells.
Figure 5.
Analysis of matriptase and HAI-1 protein expression in human breast carcinomas by immunohistochemistry. Human breast carcinomas were stained by immunohistochemistry using mAbs directed specifically against matriptase (S5) or HAI-1 (M58). Positive staining for matriptase and HAI-1 are observed as a brown precipitate (diaminobenzidine) within the sections, and nuclei were counterstained with hematoxylin. A metastatic breast adenocarcinoma shows both cytoplasmic and membranous staining for matriptase [original magnifications, ×100 (A) and ×400 (B)] and HAI-1 [original magnifications, ×100 (D) and ×400 (E)] in the breast epithelial cells. No staining is noted in stromal components of the tumor. A colloid breast carcinoma likewise shows a similar staining pattern for matriptase [original magnification, ×400 (C)] and HAI-1 [original magnification, ×400 (F)].
Figure 6.
Analysis of matriptase and HAI-1 protein expression in normal and hyperplastic human breast epithelium by immunohistochemistry. Intense staining for matriptase is seen in the duct and mild or no staining in the surrounding terminal duct lobular units (TDLU) of this area of normal breast epithelium surrounding a breast carcinoma [original magnification, ×20 (A)]. A duct with usual ductal hyperplasia shows intense staining for matriptase, and the surrounding TDLUs show mild staining [original magnification, ×40 (B)]. Focal staining for HAI-1 is noted in the TDLU, and no staining is seen in the surrounding duct [original magnification, ×40 (C)]. A high-power view of the same lobule shows preferential staining of the lobular cells, and no staining is seen in the myoepithelial cells [original magnification, ×200 (D)].
Figure 7.
Analysis of the expression of matriptase in invasive primary breast tumors by in situ hybridization. A matriptase-specific antisense probe (A and C) and the corresponding control sense probe (B and D, respectively) were hybridized to paraffin-embedded sections of primary breast tumors as described in Materials and Methods. The matriptase antisense probe shows reactivity with the cancer cells within the sections and lack of reactivity with stromal elements such as fibroblasts and adipocytes (A and C). Control sense probes do not show any reactivity with the breast cancer sections (B and D), demonstrating the specificity of the labeled antisense probe.
Figure 8.
The tissue concentration of matriptase in human breast tumors and the surrounding tissues. The tissue concentration of matriptase was determined by immunoblot. The concentration of a purified matriptase standard was determined by comparison with a bovine serum albumin standard curve resolved by SDS-polyacrylamide gel electrophoresis and stained with Coomassie Blue. Different amounts of purified matriptase were then used (80 pg, 200 pg, 400 pg, and 800 pg; lanes 1 to 4) to generate a standard curve for the immunoblot. Protein samples (50 μg) from six human breast tumors (lanes 5 to 10) and two surrounding tissues (lanes 11 and 12) were examined by immunoblot and compared to the standard curve for matriptase. The final concentration of each specimen was calculated and indicated.
Similar articles
- Expression of the serine protease matriptase and its inhibitor HAI-1 in epithelial ovarian cancer: correlation with clinical outcome and tumor clinicopathological parameters.
Oberst MD, Johnson MD, Dickson RB, Lin CY, Singh B, Stewart M, Williams A, al-Nafussi A, Smyth JF, Gabra H, Sellar GC. Oberst MD, et al. Clin Cancer Res. 2002 Apr;8(4):1101-7. Clin Cancer Res. 2002. PMID: 11948120 - Differential subcellular localization renders HAI-2 a matriptase inhibitor in breast cancer cells but not in mammary epithelial cells.
Chang HH, Xu Y, Lai H, Yang X, Tseng CC, Lai YJ, Pan Y, Zhou E, Johnson MD, Wang JK, Lin CY. Chang HH, et al. PLoS One. 2015 Mar 18;10(3):e0120489. doi: 10.1371/journal.pone.0120489. eCollection 2015. PLoS One. 2015. PMID: 25786220 Free PMC article. - Hepatocyte growth factor activation inhibitors (HAI-1 and HAI-2) regulate HGF-induced invasion of human breast cancer cells.
Parr C, Jiang WG. Parr C, et al. Int J Cancer. 2006 Sep 1;119(5):1176-83. doi: 10.1002/ijc.21881. Int J Cancer. 2006. PMID: 16557597 - Matriptase: potent proteolysis on the cell surface.
List K, Bugge TH, Szabo R. List K, et al. Mol Med. 2006 Jan-Mar;12(1-3):1-7. doi: 10.2119/2006-00022.List. Mol Med. 2006. PMID: 16838070 Free PMC article. Review. - Role of the polycystic kidney disease domain in matriptase chaperone activity and localization of hepatocyte growth factor activator inhibitor-1.
Yamashita F, Kaieda T, Shimomura T, Kawaguchi M, Lin CY, Johnson MD, Tanaka H, Kiwaki T, Fukushima T, Kataoka H. Yamashita F, et al. FEBS J. 2022 Jun;289(12):3422-3439. doi: 10.1111/febs.16348. Epub 2022 Jan 23. FEBS J. 2022. PMID: 35020274 Review.
Cited by
- E-cadherin transcriptional downregulation by promoter methylation but not mutation is related to epithelial-to-mesenchymal transition in breast cancer cell lines.
Lombaerts M, van Wezel T, Philippo K, Dierssen JW, Zimmerman RM, Oosting J, van Eijk R, Eilers PH, van de Water B, Cornelisse CJ, Cleton-Jansen AM. Lombaerts M, et al. Br J Cancer. 2006 Mar 13;94(5):661-71. doi: 10.1038/sj.bjc.6602996. Br J Cancer. 2006. PMID: 16495925 Free PMC article. - Environment-Sensitive Ectodomain Shedding of Epithin/PRSS14 Increases Metastatic Potential of Breast Cancer Cells by Producing CCL2.
Jang J, Cho EH, Cho Y, Ganzorig B, Kim KY, Kim MG, Kim C. Jang J, et al. Mol Cells. 2022 Aug 31;45(8):564-574. doi: 10.14348/molcells.2022.2004. Epub 2022 Aug 10. Mol Cells. 2022. PMID: 35950457 Free PMC article. - Mechanisms for the control of matriptase activity in the absence of sufficient HAI-1.
Xu H, Xu Z, Tseng IC, Chou FP, Chen YW, Wang JK, Johnson MD, Kataoka H, Lin CY. Xu H, et al. Am J Physiol Cell Physiol. 2012 Jan 15;302(2):C453-62. doi: 10.1152/ajpcell.00344.2011. Epub 2011 Oct 26. Am J Physiol Cell Physiol. 2012. PMID: 22031598 Free PMC article. - Activated matriptase as a target to treat breast cancer with a drug conjugate.
Rather GM, Lin SY, Lin H, Banach-Petrosky W, Hirshfield KM, Lin CY, Johnson MD, Szekely Z, Bertino JR. Rather GM, et al. Oncotarget. 2018 May 25;9(40):25983-25992. doi: 10.18632/oncotarget.25414. eCollection 2018 May 25. Oncotarget. 2018. PMID: 29899836 Free PMC article. - Targeting the membrane-anchored serine protease testisin with a novel engineered anthrax toxin prodrug to kill tumor cells and reduce tumor burden.
Martin EW, Buzza MS, Driesbaugh KH, Liu S, Fortenberry YM, Leppla SH, Antalis TM. Martin EW, et al. Oncotarget. 2015 Oct 20;6(32):33534-53. doi: 10.18632/oncotarget.5214. Oncotarget. 2015. PMID: 26392335 Free PMC article.
References
- Mignatti P, Rifkin DB: Biology and biochemistry of proteinases in tumor invasion. Physiol Rev 1993, 73:161-195 - PubMed
- Behrendt N, Ronne E, Dano K: The structure and function of the urokinase receptor, a membrane protein governing plasminogen activation on the cell surface. Biol Chem Hoppe Seyler 1995, 376:269-279 - PubMed
- Conese M, Blasi F: Urokinase/urokinase receptor system: internalization/degradation of urokinase-serpin complexes: mechanism and regulation. Biol Chem Hoppe Seyler 1995, 376:143-155 - PubMed
- Holst-Hansen C, Johannessen B, Hoyer-Hansen G, Romer J, Ellis V, Brünner N: Urokinase-type plasminogen activation in three human breast cancer cell lines correlates with their in vitro invasiveness. Clin Exp Metastasis 1996, 14:297-307 - PubMed
- Hofmann R, Lehmer A, Buresch M, Hartung R, Ulm K: Clinical relevance of urokinase plasminogen activator, its receptor, and its inhibitor in patients with renal cell carcinoma. Cancer 1996, 78:487-492 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous