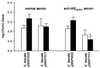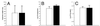Antibodies to a surface-exposed, N-terminal domain of aggregation substance are not protective in the rabbit model of Enterococcus faecalis infective endocarditis - PubMed (original) (raw)
Antibodies to a surface-exposed, N-terminal domain of aggregation substance are not protective in the rabbit model of Enterococcus faecalis infective endocarditis
J K McCormick et al. Infect Immun. 2001 May.
Abstract
The aggregation substance (AS) surface protein from Enterococcus faecalis has been implicated as an important virulence factor for the development of infective endocarditis. To evaluate the role of antibodies specific for Asc10 (the AS protein from the conjugative plasmid pCF10) in protective immunity to infective endocarditis, an N-terminal region of Asc10 lacking the signal peptide and predicted to be surface exposed (amino acids 44 to 331; AS(44-331)) was cloned with a C-terminal histidine tag translational fusion and expressed from Escherichia coli. N-terminal amino acid sequencing of the purified protein revealed the correct sequence, and rabbit polyclonal antisera raised against AS(44-331) reacted specifically to Asc10 expressed from E. faecalis OG1SSp, but not to other proteins as judged by Western blot analysis. Using these antisera, flow cytometry analysis demonstrated that antibodies to AS(44-331) bound to a surface-exposed region of Asc10. Furthermore, antibodies specific for AS(44-331) were opsonic for E. faecalis expressing Asc10 in vitro but not for cells that did not express Asc10. New Zealand White rabbits immunized with AS(44-331) were challenged intravenously with E. faecalis cells constitutively expressing Asc10 in the rabbit model of experimental endocarditis. Highly immune animals did not show significant differences in clearance of organisms from the blood or spleen or in formation of vegetations on the aortic valve, in comparison with nonimmune animals. Although in vivo expression of Asc10 was demonstrated by immunohistochemistry, these experiments provide evidence that immunity to Asc10 does not play a role in protection from experimental infective endocarditis due to E. faecalis and may have important implications for the development of immunological approaches to combat enterococcal endocarditis.
Figures
FIG. 1
Diagrammatic representation of AS44–331 cloning. Single-strand DNA sequence encoding amino acids 44 to 331 of AS (prgB) from pCF10 was amplified by PCR and cloned into pET28b. Shown is the region of prgB used for cloning and the location of the six-His sequence added to AS44–331. Underlined regions indicate the ribosomal binding site (RBS) and restriction enzymes used for cloning.
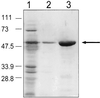
FIG. 2
SDS-PAGE analysis of purified AS44–331 protein. Purification was achieved with a nickel column with E. coli BL21(DE3) containing pJKM82 as described in Materials and Methods. Lane 1, cell extract fall through in binding buffer; lane 2, wash buffer run through the nickel column; lane 3, elution of AS44–331 from the nickel column. The band represents approximately 10 μg of protein. Numbers on the left refer to molecular masses in kilodaltons.
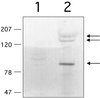
FIG. 3
Western blot analysis of surface extracts of E. faecalis OG1SSp(pMSP7517) with antiserum raised against recombinant AS44–331. Wild-type AS rapidly degrades when released from the cell, and multiple bands are common. Lane 1, uninduced culture (Asc10 negative); lane 2, Asc10 induced with 25 ng of nisin/ml. Arrows indicate bands corresponding to fragments of the Asc10 protein.
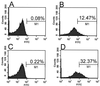
FIG. 4
Antibodies raised against the recombinant AS44–331 protein bind to the surface on E. faecalis cells only when Asc10 is expressed. Surface localization of the AS44–331 fragment was determined by flow cytometry analysis of intact E. faecalis OG1SSp(pMSP7517) cells. In experiments shown in panels A and C, Asc10 is not expressed. Expression of AS was induced (B and D) by the addition of 25 ng of nisin/ml to cultures. Panels A and B represent binding of a monoclonal antibody known to recognize surface-exposed Asc10, and panels C and D represent polyclonal antiserum raised against recombinant AS44–331. Secondary antibodies were labeled with fluorescein isothiocyanate. The percentage of gated populations is shown. Similar results were seen with E. faecalis OG1RF containing pMSP7517 (data not shown).
FIG. 5
Opsonization for intracellular killing of E. faecalis strains OG1SSp by AS44–331-specific antiserum. Strains containing pMSP7517 express Asc10, and control strains contain pMSP3535. Open bars represent bacterial counts at 0 min, and solid bars represent counts at 120 min. Data represent the average of five independent experiments. Error bars, SEM. ∗, P < 0.05 by the Student t test when compared with all other bacterial counts at 120 min. There was no significant difference among bacterial counts at 0 min.
FIG. 6
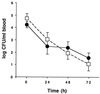
Bacteremia in AS44–331-immune (●) and nonimmune (□) New Zealand White rabbits challenged with E. faecalis OG1SSp containing pINY1801 over 72 h. For each rabbit, approximately 2 × 109 CFU of stationary-phase E. faecalis OG1SSp containing pINY1801 was initially administered by the marginal ear vein. Blood samples were drawn daily and plated on TH agar to determine counts. Data are the averages for seven rabbits per group. Error bars, SEM.
FIG. 7
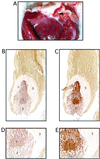
Vegetation formation on rabbit heart valve tissue by E. faecalis OG1SSp containing pINY1801 and demonstration of in vivo expression of Asc10. A typical vegetation formation on the rabbit aortic valve is shown in panel A. The arrowhead indicates the vegetation located within one of the aortic valves. Panels B and C (magnification, ×40) and D and E (magnification, ×200) represent consecutive 5-μm sections of a vegetation stained with preimmune serum (B and D) and AS44–331-immune serum (C and E). The secondary antibody was anti-rabbit horseradish peroxidase conjugate, and sections were developed with 3,3-diaminobenzidine as chromogen (brown color). Regions of the vegetation are identified as follows: a mass of E. faecalis cells (1) bordered by an influx of immune cells (2) and surrounded by a platelet/fibrin layer (3). Slides were counterstained with hematoxlyin.
FIG. 8
Comparison between AS44–331-immune (solid bars) and nonimmune (open bars) New Zealand White rabbits challenged with E. faecalis OG1SSp containing pINY1801. Immune animals were immunized with 25 μg of purified AS44–331 protein in Freund's incomplete adjuvant at 2-week intervals for a total of three immunizations. After 72 h, hearts were resected, vegetations were weighed (A) and homogenized, and bacterial counts were quantitatively determined (B). Spleens were also aseptically harvested, weighed, and homogenized, and bacterial counts were quantitatively determined (C). Data represent the average for seven rabbits per group. Error bars, SEM.
Similar articles
- Multiple functional domains of Enterococcus faecalis aggregation substance Asc10 contribute to endocarditis virulence.
Chuang ON, Schlievert PM, Wells CL, Manias DA, Tripp TJ, Dunny GM. Chuang ON, et al. Infect Immun. 2009 Jan;77(1):539-48. doi: 10.1128/IAI.01034-08. Epub 2008 Oct 27. Infect Immun. 2009. PMID: 18955479 Free PMC article. - Formation of vegetations during infective endocarditis excludes binding of bacterial-specific host antibodies to Enterococcus faecalis.
McCormick JK, Tripp TJ, Dunny GM, Schlievert PM. McCormick JK, et al. J Infect Dis. 2002 Apr 1;185(7):994-7. doi: 10.1086/339604. Epub 2002 Mar 11. J Infect Dis. 2002. PMID: 11920326 - An amino-terminal domain of Enterococcus faecalis aggregation substance is required for aggregation, bacterial internalization by epithelial cells and binding to lipoteichoic acid.
Waters CM, Hirt H, McCormick JK, Schlievert PM, Wells CL, Dunny GM. Waters CM, et al. Mol Microbiol. 2004 May;52(4):1159-71. doi: 10.1111/j.1365-2958.2004.04045.x. Mol Microbiol. 2004. PMID: 15130132 - Rationale for the development of immunotherapy regimens against enterococcal infections.
Theilacker C, Krueger WA, Kropec A, Huebner J. Theilacker C, et al. Vaccine. 2004 Dec 6;22 Suppl 1:S31-8. doi: 10.1016/j.vaccine.2004.08.014. Vaccine. 2004. PMID: 15576199 Review. - Vancomycin-resistant Enterococcus faecalis endocarditis: linezolid failure and strain characterization of virulence factors.
Tsigrelis C, Singh KV, Coutinho TD, Murray BE, Baddour LM. Tsigrelis C, et al. J Clin Microbiol. 2007 Feb;45(2):631-5. doi: 10.1128/JCM.02188-06. Epub 2006 Dec 20. J Clin Microbiol. 2007. PMID: 17182759 Free PMC article. Review.
Cited by
- Insights into ecology, pathogenesis, and biofilm formation of Enterococcus faecalis from functional genomics.
Willett JLE, Dunny GM. Willett JLE, et al. Microbiol Mol Biol Rev. 2025 Mar 27;89(1):e0008123. doi: 10.1128/mmbr.00081-23. Epub 2024 Dec 23. Microbiol Mol Biol Rev. 2025. PMID: 39714182 Review. - Glucosyltransferases of viridans group streptococci modulate interleukin-6 and adhesion molecule expression in endothelial cells and augment monocytic cell adherence.
Yeh CY, Chen JY, Chia JS. Yeh CY, et al. Infect Immun. 2006 Feb;74(2):1273-83. doi: 10.1128/IAI.74.2.1273-1283.2006. Infect Immun. 2006. PMID: 16428777 Free PMC article. - Importance of the collagen adhesin ace in pathogenesis and protection against Enterococcus faecalis experimental endocarditis.
Singh KV, Nallapareddy SR, Sillanpää J, Murray BE. Singh KV, et al. PLoS Pathog. 2010 Jan 8;6(1):e1000716. doi: 10.1371/journal.ppat.1000716. PLoS Pathog. 2010. PMID: 20072611 Free PMC article. - Acceleration of Enterococcus faecalis biofilm formation by aggregation substance expression in an ex vivo model of cardiac valve colonization.
Chuang-Smith ON, Wells CL, Henry-Stanley MJ, Dunny GM. Chuang-Smith ON, et al. PLoS One. 2010 Dec 30;5(12):e15798. doi: 10.1371/journal.pone.0015798. PLoS One. 2010. PMID: 21209892 Free PMC article. - Model systems for the study of Enterococcal colonization and infection.
Goh HMS, Yong MHA, Chong KKL, Kline KA. Goh HMS, et al. Virulence. 2017 Nov 17;8(8):1525-1562. doi: 10.1080/21505594.2017.1279766. Epub 2017 May 4. Virulence. 2017. PMID: 28102784 Free PMC article. Review.
References
- Almirante B, Tornos M P, Gurgui M, Pujol M, Miro J M. Prognosis of enterococcal endocarditis. Rev Infect Dis. 1991;13:1248–1249. - PubMed
- Andrewes F W, Horder T J. A study of the streptococci pathogenic for man. Lancet. 1906;ii:708–713.
- Berti M, Candiani G, Kaufhold A, Muscholl A, Wirth R. Does aggregation substance of Enterococcus faecalis contribute to development of endocarditis? Infection. 1998;26:48–53. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials