Cadherin sequences that inhibit beta-catenin signaling: a study in yeast and mammalian cells - PubMed (original) (raw)
Cadherin sequences that inhibit beta-catenin signaling: a study in yeast and mammalian cells
I Simcha et al. Mol Biol Cell. 2001 Apr.
Free PMC article
Abstract
Drosophila Armadillo and its mammalian homologue beta-catenin are scaffolding proteins involved in the assembly of multiprotein complexes with diverse biological roles. They mediate adherens junction assembly, thus determining tissue architecture, and also transduce Wnt/Wingless intercellular signals, which regulate embryonic cell fates and, if inappropriately activated, contribute to tumorigenesis. To learn more about Armadillo/beta-catenin's scaffolding function, we examined in detail its interaction with one of its protein targets, cadherin. We utilized two assay systems: the yeast two-hybrid system to study cadherin binding in the absence of Armadillo/beta-catenin's other protein partners, and mammalian cells where interactions were assessed in their presence. We found that segments of the cadherin cytoplasmic tail as small as 23 amino acids bind Armadillo or beta-catenin in yeast, whereas a slightly longer region is required for binding in mammalian cells. We used mutagenesis to identify critical amino acids required for cadherin interaction with Armadillo/beta-catenin. Expression of such short cadherin sequences in mammalian cells did not affect adherens junctions but effectively inhibited beta-catenin-mediated signaling. This suggests that the interaction between beta-catenin and T cell factor family transcription factors is a sensitive target for disruption, making the use of analogues of these cadherin derivatives a potentially useful means to suppress tumor progression.
Figures
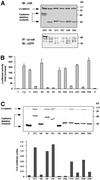
Figure 1
Mapping the minimal binding site on DEC cytoplasmic tail for β-catenin (βcat) and Arm using the yeast two-hybrid (2 hyb) system. (A) Schematic representation of the DEC derivatives used in our analyses, with ability to bind Arm/β-catenin in either yeast or mammalian cells summarized in the right-hand columns. TM, transmembrane; TC, tissue culture. *Data from Pai_et al._ (1996). (B) Sequence of the minimal binding region of DE-cadherin, with the boundaries of the smallest DEC derivatives indicated. (C) All of the DEC derivatives bind to both fragments of Arm and β-catenin in yeast. The full-length DE-cadherin cytoplasmic domain (DEC), or smaller derivatives of DEC (diagrammed in A and B), fused to the Gal4 transcriptional activation domain, were transformed into yeast cells along with portions of Arm or β-catenin fused to the LexA DNA-binding domain. Average β-galactosidase values are shown for each DEC derivative together with the full Arm repeat region of Arm or β-catenin (Arm R1–12 or βcat R1–12, left), or a smaller fragment of the Arm repeat region (Arm R2–10 or βcat R2–10, right). 0, background level of β-galactosidase activity with no DEC fragment fused to Gal4. **DEC 25 was tested against only Arm R1–12. Its β-galactosidase value was 14.4 U, compared with 18.3 U for the negative control.
Figure 2
Analysis of the ability of different fragments of the DEC cytoplasmic tail to interact with β-catenin, affect its stability, and inhibit β-catenin–mediated transactivation. (A) The ability of selected GFP-DEC derivatives to coimmunoprecipitate with cotransfected HA-tagged β-catenin was determined by immunoprecipitation (IP) from 293T cells transfected with HA-tagged β-catenin and GFP-tagged DEC constructs with anti-GFP antibody, followed by Western blotting with anti-HA antibody. The total level of transfected β-catenin and DEC constructs was determined by immunoblotting (IB) with anti-HA-antibody. (B) 293T cells were transfected with GFP-tagged derivatives of the DEC cytoplasmic tail (DEC) or the full-length mammalian E-cadherin tail (E), along with β-catenin (β), a LEF/TCF reporter plasmid (T), and Lac Z. Luciferase activity was determined from duplicate plates as fold activation after normalizing for transfection efficiency by measuring β-galactosidase activity. T, cells were transfected with the reporter plasmid alone; V, cells transfected with the reporter plasmid, HA-tagged β-catenin and the GFP-vector used for the construction of the cadherin derivatives. (C) The cadherin derivatives used in B were transfected into CHO cells, and their ability to protect the endogenous β-catenin from degradation was determined by analyzing the level of β-catenin expressed in the DEC mutant-transfected cells by Western blotting with anti-β-catenin antibody. The level of expression of DEC constructs was determined by immunoblotting with an antibody against the GFP tag. Quantitation of the β-catenin level expressed in CHO cells was carried out by normalizing the intensity of the β-catenin bands shown to those of the DEC band for each derivative.
Figure 3
The effect of DEC cytoplasmic domain derivatives on the organization of adherens junctions and subcellular distribution of β-catenin (β-cat). MDCK cells were transfected with various GFP-tagged DEC derivatives (diagrammed in Figure 1, A and B), and the distribution of the GFP-tagged DEC derivatives (A, C, E, G, and I) and of the endogenous β-catenin (B, D, F, H, and J) was determined by double fluorescence microscopy using rhodamine-labeled anti-β-catenin antibody. Bar (in A), 10 μm. Note the reduction in junctional β-catenin in DEC-expressing cells but not in cells transfected with other DEC constructs. Also note that DEC9 and DEC29 do not increase the endogenous β-catenin level, whereas DEC13 does.
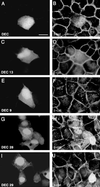
Figure 4
Analysis of the ability of DEC derivatives to increase the level and the accumulation of endogenous β-catenin (β-cat) in the nucleus. Some of the GFP-DEC constructs described in Figure 3 were transfected into CHO cells (A, C, and E), and their ability to elevate the endogenous β-catenin and induce its translocation into the nucleus (B, D, and F) was determined by double fluorescence as described in Figure 3. Bar in (A), 10 μm. The arrows mark the transfected cells. Note that, whereas DEC13 and DEC induced the accumulation of endogenous β-catenin in the nucleus, DEC9 was unable to do so.
Figure 5
Analysis of the effect of clustered point mutations in the minimal Arm-binding domain of DEC on its ability to bind Arm and β-catenin (βcat) in the yeast two-hybrid system. (A) Diagram of the DEC tail and sequences of the clustered point mutations used in this study, with the sequences of DE-cadherin (DE-Cad) and human E-cadherin (hE-Cad) in the region of the mutations shown below. All mutations were introduced into and analyzed in the context of the full-length cytoplasmic tail. The mutation DECM2 was also tested in the context of a smaller fragment of the cadherin tail (DEC30; Figure 1A)—this derivative is DEC30(M2). (B) The DE-cadherin mutants diagrammed in A were fused to the Gal4 transcription activation domain and transformed into yeast cells together with the full Arm repeat region of Arm or β-catenin (Arm R1–12 or βcat R1–12, left) or a smaller fragment of the Arm repeat region (Arm R2–10 or βcat R2–10, right), fused to the LexA DNA-binding domain. Average β-galactosidase activities are shown.
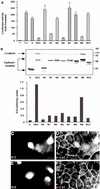
Figure 6
Analysis of the effect of clustered point mutations in the minimal Arm-binding domain of DEC on its capacity to interact with β-catenin, protect it from degradation, inhibit β-catenin/LEF-mediated transactivation, and affect β-catenin organization. (A) The ability of clustered point mutations (diagrammed in Figure 5A) to affect β-catenin/LEF-1–mediated transactivation in 293T cells was examined as described in Figure 2B. (B) The ability to protect β-catenin from degradation was examined in CHO cells, and the levels of β-catenin were quantified as described in Figure 2C. Because the samples were originally analyzed on the same gel with the samples shown in Figure 2C, the control samples (V, DEC, and D9) are shown again. (C–F) MDCK cells were transfected with GFP-tagged DECM3 (C, M3) and DECM8 (E, M8), and the organization of the endogenous β-catenin (β-cat) in the respective samples (D and F) was determined by double fluorescence microscopy. Bar, 10 μm.
Figure 7
A model for the structure of the β-catenin-binding region of cadherin. (A) The sequence of_Xenopus_ Tcf3 (XTcf3) and Drosophila TCF (dTCF) are aligned below a diagrammatic representation of the structure of XTcf3 as determined by Graham et al. (2000). A β-hairpin motif is indicated by “β ->.” Identical residues are indicated by vertical lines and similar residues are indicated by colons. Below is a proposed alignment of Drosophila E-cadherin (DE-cad) and mouse E-cadherin (mE-cad) with the extended peptide and α-helical regions of XTcf3. A consensus is displayed at the bottom positions (where at least three fourths of the sequences match). The three residues that are key for XTcf3 binding to β-catenin are boxed, and all are conserved in all sequences. The amino acids altered in mutation DECM2 (YEG G), which have a strong effect on DEC binding to β-catenin/Arm in our assays, are bold and underlined. The amino acids altered in mutation DECM3 (DD D), which had the weakest effect on binding, are shown in italics. The serines altered in mutations DECM1 and DECM9 are in italics and underlined. (B) Alignment of the XTcf3 sequence and structure with four 20-amino acid repeats of Drosophila APC (dAPC).
Similar articles
- Drosophila alpha-catenin and E-cadherin bind to distinct regions of Drosophila Armadillo.
Pai LM, Kirkpatrick C, Blanton J, Oda H, Takeichi M, Peifer M. Pai LM, et al. J Biol Chem. 1996 Dec 13;271(50):32411-20. doi: 10.1074/jbc.271.50.32411. J Biol Chem. 1996. PMID: 8943306 - Signaling and adhesion activities of mammalian beta-catenin and plakoglobin in Drosophila.
White P, Aberle H, Vincent JP. White P, et al. J Cell Biol. 1998 Jan 12;140(1):183-95. doi: 10.1083/jcb.140.1.183. J Cell Biol. 1998. PMID: 9425166 Free PMC article. - Armadillo and dTCF: a marriage made in the nucleus.
Cavallo R, Rubenstein D, Peifer M. Cavallo R, et al. Curr Opin Genet Dev. 1997 Aug;7(4):459-66. doi: 10.1016/s0959-437x(97)80071-8. Curr Opin Genet Dev. 1997. PMID: 9309175 Review. - TCF/LEF factor earn their wings.
Clevers H, van de Wetering M. Clevers H, et al. Trends Genet. 1997 Dec;13(12):485-9. doi: 10.1016/s0168-9525(97)01305-x. Trends Genet. 1997. PMID: 9433138 Review.
Cited by
- The N-cadherin cytoplasmic domain confers anchorage-independent growth and the loss of contact inhibition.
Ozawa M. Ozawa M. Sci Rep. 2015 Oct 20;5:15368. doi: 10.1038/srep15368. Sci Rep. 2015. PMID: 26481443 Free PMC article. - Cadherins mediate both the association between PS1 and beta-catenin and the effects of PS1 on beta-catenin stability.
Serban G, Kouchi Z, Baki L, Georgakopoulos A, Litterst CM, Shioi J, Robakis NK. Serban G, et al. J Biol Chem. 2005 Oct 28;280(43):36007-12. doi: 10.1074/jbc.M507503200. Epub 2005 Aug 26. J Biol Chem. 2005. PMID: 16126725 Free PMC article. - A polycystin-1 multiprotein complex is disrupted in polycystic kidney disease cells.
Roitbak T, Ward CJ, Harris PC, Bacallao R, Ness SA, Wandinger-Ness A. Roitbak T, et al. Mol Biol Cell. 2004 Mar;15(3):1334-46. doi: 10.1091/mbc.e03-05-0296. Epub 2004 Jan 12. Mol Biol Cell. 2004. PMID: 14718571 Free PMC article. - M-cadherin-inhibited phosphorylation of ß-catenin augments differentiation of mouse myoblasts.
Wang Y, Mohamed JS, Alway SE. Wang Y, et al. Cell Tissue Res. 2013 Jan;351(1):183-200. doi: 10.1007/s00441-012-1515-4. Epub 2012 Nov 9. Cell Tissue Res. 2013. PMID: 23138569 Free PMC article. - Promoter characterization of the novel human matrix metalloproteinase-26 gene: regulation by the T-cell factor-4 implies specific expression of the gene in cancer cells of epithelial origin.
Marchenko GN, Marchenko ND, Leng J, Strongin AY. Marchenko GN, et al. Biochem J. 2002 Apr 15;363(Pt 2):253-62. doi: 10.1042/0264-6021:3630253. Biochem J. 2002. PMID: 11931652 Free PMC article.
References
- Ben-Ze'ev A, Geiger B. Differential molecular interactions of β-catenin and plakoglobin in adhesion, signaling and cancer. Curr Opin Cell Biol. 1998;10:629–639. - PubMed
- Christofori G, Semb H. The role of the cell-adhesion molecule E-cadherin as a tumor-suppressor gene. Trends Biochem Sci. 1999;24:73–76. - PubMed
- Conti E, Uy M, Leighton L, Blobel G, Kuriyan J. Crystallographic analysis of the recognition of a nuclear localization signal by the nuclear import factor karyopherin-α. Cell. 1998;94:193–204. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous