Involvement of the ERK mitogen-activated protein kinase in cell resistance to complement-mediated lysis - PubMed (original) (raw)
Involvement of the ERK mitogen-activated protein kinase in cell resistance to complement-mediated lysis
S Kraus et al. Clin Exp Immunol. 2001 Mar.
Abstract
Sublytic doses of complement desensitize cells and make them resistant to lytic complement doses. This process, named complement-induced protection, requires calcium ion influx, protein kinase C activation and protein synthesis. The involvement of the extracellular signal-regulated kinase, ERK, in cell desensitization by sublytic complement was examined in erythroleukaemia K562 cells and in COS-7 cells. As shown here, ERK is activated in K562 and COS-7 cells within 10 min of sublytic immune attack and then shows a decline and a second peak of activation at 20 min. C7- and C8-deficient human sera have a small effect on ERK activity. However, a significant increase in ERK activation is observed when C7 or C8, respectively, is added back to these sera. Complement-induced ERK activation was blocked in cells treated with GF109203X or Go6976, two selective PKC inhibitors, as well as by treatment with PD098059, an inhibitor of MEK1, the ERK kinase. PD098059 treatment also sensitized K562 cells to complement-mediated lysis and prevented complement-induced protection. COS-7 cells transfected with a dominant-negative MEK plasmid were incapable of undergoing the process of complement-induced protection. In conclusion, cell desensitization by sublytic doses of the complement membrane attack complex involves a signalling cascade that includes PKC-mediated ERK activation.
Figures
Fig. 1
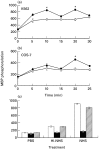
Complement-induced MAPK activation in (a) K562 and (b) COS-7 cells. (a,b) K562 and COS-7 cells were serum-starved by growth in RPMI or DMEM, respectively, in presence of 0·1% HI-FCS for 18 h in a 5% CO2 incubator and then treated for 30 min on ice with antibody (diluted 1 : 25) and then with complement (HI-NHS (○) or NHS (•) diluted 1 : 2) at 37°C for 5–30 min. Cells treated with PBS served to determine basal MAPK activity (Time 0). Following incubation, cell extracts were prepared and fractionated. MAPK activity was determined in triplicates by MBP phosphorylation. The results are representative of 4 independent experiments. (c) Serum-starved K562 cells were preincubated without (control □) or with 1 µ
m
PD098059 (▪) or 0·02% DMSO ( ) (60 min, 37°C) and then treated with sublytic concentrations of antibody and complement (HI-NHS or NHS) for 10 min at 37°C. MAPK activity was determined as above. Results are representative of 3 independent experiments. *P < 0·01, **P < 0·001.
) (60 min, 37°C) and then treated with sublytic concentrations of antibody and complement (HI-NHS or NHS) for 10 min at 37°C. MAPK activity was determined as above. Results are representative of 3 independent experiments. *P < 0·01, **P < 0·001.
Fig. 2
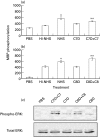
ERK1,2 activation by reconstituted C7-and C8-deficient serum. Serum-starved and antibody coated K562 cells were stimulated for 10 min at 37°C with complement: (a) HI-NHS, NHS, C7-deficient human serum (C7D) and C7D supplemented with C7; (b) HI-NHS, NHS, C8-deficient human serum (C8D) and C8D supplemented with C8; (c) C7D or C8D with or without C7 or C8, respectively. PBS-treated cells served as control. (a,b): MAPK activity was determined in cell extracts by MBP phosphorylation. Statistical significance was analysed by the student _t_-test. * NHS versus HI-NHS; P < 0·01. ** C7D + C7 versus C7D; P < 0·05. ** C8D + C8 versus C8D; P < 0·001. (c) Active and total ERK levels were examined by Western Blotting with anti‐phospho ERK and anti-ERK antibodies.
Fig. 3
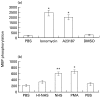
Effect of calcium ionophores and PMA on MAPK activity. Serum-starved K562 cells were treated with (a) ionophores: 3 µ
m
ionomycin, 25 µ
m
A23187 or 0·01% DMSO (b) antibody and complement (HI-NHS or NHS) or PMA (10 µg/ml) or DMSO (0·01%) for 10 min at 37°C. PBS-treated cells served as control. Statistical significance of differences (ionophores or PMA versus DMSO; NHS versus HI-NHS) was analysed by the student _t_-test. * P < 0·001, ** P < 0·01. Results are representative of 3 independent experiments.
Fig. 4
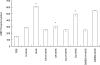
Effect of PKC inhibitors on complement-induced MAPK activity. Serum-starved K562 cells were preincubated with or without GF109203X (0·5 µ
m
) or Go6976 (2 n
m
) or DMSO (0·5%) for 60 min at 37°C and then treated with sublytic concentrations of antibody and complement (HI-NHS or NHS) for 10 min at 37°C. PBS-treated cells served as control. Statistical significance was analysed by the student _t_-test. * NHS versus HI-NHS, GF109203X + NHS versus NHS, Go6976 + NHS versus NHS or versus DMSO + NHS; P < 0·001. Results are representative of 4 independent experiments.
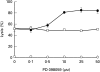
Fig. 5
Effect of PD098059 on complement-mediated cell lysis. K562 cells were pretreated with increasing concentrations of PD098059 or DMSO for 60 min at 37°C and then incubated with anti-K562 antibodies (30 min, 4°C) and with HI-NHS or NHS (1 : 2) for 60 min at 37°C. Percent lysis was determined in triplicates by trypan blue exclusion. Results are expressed as the mean percent lysis ± SD. Results are representative of 3 independent experiments. ○ PD098059 + HI-NHS; • PD098059 + NHS; □ DMSO+NHS.
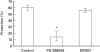
Fig. 6
Effect of PD098059 on complement-induced protection. K562 cells were pretreated without (control) or with: PD098059 (1 µ
m
) or DMSO (0·02%) for 60 min at 37°C and then treated with sublytic antibody and HI-NHS or NHS. Next the cells were subjected to lytic antibody and NHS diluted 1:8 in PBS. Percent protection in induced cells (treated with NHS) was calculated relative to noninduced cells (treated with HI-NHS). Results are expressed as the mean percent protection ± SD (n = 4). The experiment is representative of 3 independent experiments. * P < 0·001.
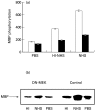
Fig. 7
Complement-induced MAPK activity in COS-7 cells transfected with dominant-negative MEK. Serum-starved COS-7 cells 48 h after transfection with DN-MEK mutant (▪) and nontransfected cells (□ Control) were treated with sublytic concentrations of antibody and complement (HI-NHS or NHS) for 20 min at 37°C. PBS-treated cells were used as control. (a) MAPK activity was analysed by MBP phosphorylation (b) MAPK activity was assayed by phosphorylation of MBP after immunoprecipitation of cell extracts with anti ERK C-terminus antibodies and protein A-Sepharose. The immunoprecipitates were subjected to SDS-PAGE in a 15% gel. PBS-treated cells were used as control. Autoradiogram of phosphorylated MBP is shown.
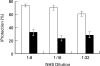
Fig. 8
Effect of DN-MEK on complement-induced protection in COS-7 cells. COS-7 cells after transfection (▪) or nontransfected cells (□) were treated with a sublytic dose of antibody and HI-NHS or NHS. Next, the cells were subjected to lytic antibody and NHS serially diluted 1:8–1:32 in PBS. Percent protection in induced cells (treated with NHS) was calculated relative to noninduced cells (treated with HI-NHS). Results are expressed as the mean percent protection + SD (n = 3). The experiment is representative of 3 independent experiments.
Similar articles
- Cell desensitization by sublytic C5b-9 complexes and calcium ionophores depends on activation of protein kinase C.
Kraus S, Fishelson Z. Kraus S, et al. Eur J Immunol. 2000 May;30(5):1272-80. doi: 10.1002/(SICI)1521-4141(200005)30:5<1272::AID-IMMU1272>3.0.CO;2-9. Eur J Immunol. 2000. PMID: 10820372 - Complement membrane attack complex, perforin, and bacterial exotoxins induce in K562 cells calcium-dependent cross-protection from lysis.
Reiter Y, Ciobotariu A, Jones J, Morgan BP, Fishelson Z. Reiter Y, et al. J Immunol. 1995 Aug 15;155(4):2203-10. J Immunol. 1995. PMID: 7636268 - Sublytic complement attack protects tumor cells from lytic doses of antibody and complement.
Reiter Y, Ciobotariu A, Fishelson Z. Reiter Y, et al. Eur J Immunol. 1992 May;22(5):1207-13. doi: 10.1002/eji.1830220515. Eur J Immunol. 1992. PMID: 1577063 - Extracellular signal-regulated kinase, Jun N-terminal kinase, p38, and c-Src are involved in gonadotropin-releasing hormone-stimulated activity of the glycoprotein hormone follicle-stimulating hormone beta-subunit promoter.
Bonfil D, Chuderland D, Kraus S, Shahbazian D, Friedberg I, Seger R, Naor Z. Bonfil D, et al. Endocrinology. 2004 May;145(5):2228-44. doi: 10.1210/en.2003-1418. Epub 2004 Jan 21. Endocrinology. 2004. PMID: 14736735 - Complement resistance of tumor cells: basal and induced mechanisms.
Jurianz K, Ziegler S, Garcia-Schüler H, Kraus S, Bohana-Kashtan O, Fishelson Z, Kirschfink M. Jurianz K, et al. Mol Immunol. 1999 Sep-Oct;36(13-14):929-39. doi: 10.1016/s0161-5890(99)00115-7. Mol Immunol. 1999. PMID: 10698347 Review.
Cited by
- EGFR modulates complement activation in head and neck squamous cell carcinoma.
Abu-Humaidan AHA, Ekblad L, Wennerberg J, Sørensen OE. Abu-Humaidan AHA, et al. BMC Cancer. 2020 Feb 13;20(1):121. doi: 10.1186/s12885-020-6615-z. BMC Cancer. 2020. PMID: 32054454 Free PMC article. - Identification of a central role for complement in osteoarthritis.
Wang Q, Rozelle AL, Lepus CM, Scanzello CR, Song JJ, Larsen DM, Crish JF, Bebek G, Ritter SY, Lindstrom TM, Hwang I, Wong HH, Punzi L, Encarnacion A, Shamloo M, Goodman SB, Wyss-Coray T, Goldring SR, Banda NK, Thurman JM, Gobezie R, Crow MK, Holers VM, Lee DM, Robinson WH. Wang Q, et al. Nat Med. 2011 Nov 6;17(12):1674-9. doi: 10.1038/nm.2543. Nat Med. 2011. PMID: 22057346 Free PMC article. - Longitudinally extensive NMO spinal cord pathology produced by passive transfer of NMO-IgG in mice lacking complement inhibitor CD59.
Zhang H, Verkman AS. Zhang H, et al. J Autoimmun. 2014 Sep;53:67-77. doi: 10.1016/j.jaut.2014.02.011. Epub 2014 Mar 31. J Autoimmun. 2014. PMID: 24698947 Free PMC article. - TNF-α promotes human antibody-mediated complement-dependent cytotoxicity of porcine endothelial cells through downregulating P38-mediated Occludin expression.
Gao H, Cao M, Chen P, Cooper DKC, Zhao Y, Wei L, Xu J, Cai Z, Zeng C, Luan S, Mou L. Gao H, et al. Cell Commun Signal. 2019 Jul 15;17(1):75. doi: 10.1186/s12964-019-0386-7. Cell Commun Signal. 2019. PMID: 31307477 Free PMC article. - Sublytic complement protects prostate cancer cells from tumour necrosis factor-α-induced cell death.
Liu L, Li W, Li Z, Kirschfink M. Liu L, et al. Clin Exp Immunol. 2012 Aug;169(2):100-8. doi: 10.1111/j.1365-2249.2012.04596.x. Clin Exp Immunol. 2012. PMID: 22774984 Free PMC article.
References
- Muller-Eberhard HJ. The membrane attack complex of complement. Ann Rev Immunol. 1986;4:503–428. - PubMed
- Jurianz K, Ziegler S, Garcia-Schuler H, et al. Complement resistance of tumor cells: basal and induced mechanisms. Mol Immunol. 1999;36:929–39. - PubMed
- Morgan BP, Meri S. Membrane Proteins That Protect Against Complement Lysis. Springer SeminImmunopathol. 1994;15:369–96. - PubMed
- Ollert MW, Frade R, Fiandino A, et al. C3- cleaving membrane proteinase: a new complement regulatory protein of human melanoma cells. J Immunol. 1990;144:3862–7. - PubMed
- Paas Y, Bohana-Kashtan O, Fishelson Z. Phosphorylation of the complement component, C9, by an ecto-protein kinase of human leukemic cells. Immunopharmacology. 1999;42:175–85. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous