Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques: implications for the neuropathogenesis of AIDS - PubMed (original) (raw)
Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques: implications for the neuropathogenesis of AIDS
K C Williams et al. J Exp Med. 2001.
Abstract
The macrophage is well established as a target of HIV and simian immunodeficiency virus (SIV) infection and a major contributor to the neuropathogenesis of AIDS. However, the identification of distinct subpopulations of monocyte/macrophages that carry virus to the brain and that sustain infection within the central nervous system (CNS) has not been examined. We demonstrate that the perivascular macrophage and not the parenchymal microglia is the primary cell productively infected by SIV. We further demonstrate that although productive viral infection of the CNS occurs early, thereafter it is not easily detectable until terminal AIDS. The biology of perivascular macrophages, including their rate of turnover and replacement by peripheral blood monocytes, may explain the timing of neuroinvasion, disappearance, and reappearance of virus in the CNS, and questions the ability of the brain to function as a reservoir for productive infection by HIV/SIV.
Figures
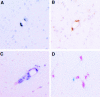
Figure 1
CD11b immunoreactivity in the CNS of normal noninfected and SIV-infected macaques. (A) CD11b immunoreactivity on nonactivated parenchymal microglia with branched and crenulated processes in a normal noninfected animal. These cells makeup a reticular branching network throughout the white matter in the CNS. (B) CD11b immunoreactivity on parenchymal microglia in the CNS of an animal with SIVE. Microglia maintain a reticular network in the CNS but morphologically become more rounded with fewer processes, consistent with cellular activation.
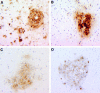
Figure 2
Immunophenotype of parenchymal microglia and perivascular macrophages in macaques with SIVE. (A) CD11b immunoreactivity on brain macrophages. Immunoreactivity is demonstrated on parenchymal microglia evenly spaced throughout the white matter, and on expansive aggregates of perivascular macrophages and MNGCs typical of SIVE. (B) CD14 immunoreactivity on perivascular macrophages within a SIVE lesion. CD14 is not detected on parenchymal microglia. (C) CD45 immunoreactivity on perivascular macrophages and lymphocytes within an SIVE lesion. CD45 is not detected on parenchymal microglia. (D) Viral envelope protein (gp120) in SIVE. The distribution of SIV-gp120 appears to overlap with the distribution of CD11b+CD14+CD45+ perivascular macrophages (A–C). Data presented here are representative of immunohistochemical studies performed on two to three CNS regions of n = 5 animals with SIVE.
Figure 3
Perivascular macrophages are infected in the CNS of animals at peak viremia. (A) SIV in situ hybridization–positive cells in the CNS in a perivascular location. (B) CD14 immunoreactivity on a serial section demonstrating that the in situ–positive cells shown in A are CD14-positive perivascular macrophages. (C) Double-label immunohistochemistry for detection of CD14 (red) and viral protein (gp120; blue). Essentially all of the cells in this perivascular cuff are CD14+gp120+ (purple) perivascular macrophages. (D) Double-label immunohistochemistry on a tissue section from the thymus of an SIV-infected animal demonstrating single-color controls using a macrophage marker (red) and anti-SIV–gp120 (blue). Thymus tissues as single color controls were performed in parallel with all double-label immunohistochemistry experiments. Results are representative of immunohistochemical studies performed on two to three CNS regions of n = 5 animals killed at peak viremia.
Figure 4
Perivascular macrophages are the major target of SIV in animals with SIVE. Double-label immunohistochemistry for CD14 (red) and viral protein (gp120; blue). Colocalization results in purple cells. (A) Numerous CD14+ perivascular macrophages within an SIVE lesion. Few of the CD14+ cells (red) in this lesion are infected as demonstrated by the small number of purple cells. (B) Numerous CD14+ perivascular macrophages are viral infected (purple) in a different lesion in the CNS of the same animal. (C) A MNGC that is CD14+ and gp120+ (purple) suggesting that MNGCs arise from the fusion of CD14+ perivascular macrophages and not CD14− parenchymal microglia. Results are representative of immunohistochemical studies performed on two to three CNS regions of n = 5 animals with SIVE.
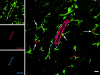
Figure 5
Triple-label confocal microscopy of the brain of an SIV-infected macaque. Images for individual channels (CD11b, green; Glut-1, red; and CD14, blue) are shown on the left and a larger merged image contain all three channels plus the differential interference contrast (DIC) image are shown on the right. Parenchymal microglia (CD11b, green) maintain a reticular network in the white matter of SIV-infected macaques. Perivascular macrophages (CD11b+CD14+, blue-green) are situated around CNS endothelium (Glut-1, red). Both parenchymal microglia (green) and perivascular macrophages (blue-green) are in contact with CNS endothelium where parenchymal microglia have foot processes on endothelium (arrows) and perivascular macrophages are in contact with and wrap around CNS vessels. Bar, 10 μM.
Figure 6
Accumulation of perivascular macrophages in the CNS of SIV-infected macaques. Images for individual channels (CD11b, green; Glut-1, red; and CD14, blue) are shown on the left and the merged image combining all three channels plus the DIC image are shown on the right. CD11b+ (green) and CD14+ (blue) perivascular macrophages (blue-green) accumulate around a CNS vessel (Glut-1, red) in an SIVE lesion. Parenchymal microglia that are CD11b+CD14− (green) outside of the lesion area maintain a reticular network, although their morphology has changed as a result of activation. Bar, 10 μM.
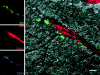
Figure 7
SIV-infected perivascular macrophages. Images for individual channels (SIV-p28, green; Glut-1, red; and CD14, blue) are shown on the left, and the merged image combining all three channels plus the DIC image is shown on the right. Perivascular macrophages (CD14, blue) that are viral protein positive (SIV-p28, green) appear blue-green near a CNS vessel (Glut-1, red). Bar, 10 μM.
Figure 8
CNS macrophages are the target of SIV infection in animals with SIVE. CNS macrophages (CD11b, green), astrocytes (GFAP, yellow), CNS endothelial cells (Glut-1, red), and viral protein (SIV-p28, blue) were examined to assess whether cells other than macrophages were productively infected. The image shows a combination of the four channels plus the DIC image. Extensive analysis of multiple brain regions from n = 5 animals with SIVE demonstrated p28-positive infected cells were exclusively CD11b+ macrophages. Bar, 10 μM.
Similar articles
- Neuroinvasion by simian immunodeficiency virus coincides with increased numbers of perivascular macrophages/microglia and intrathecal immune activation.
Lane JH, Sasseville VG, Smith MO, Vogel P, Pauley DR, Heyes MP, Lackner AA. Lane JH, et al. J Neurovirol. 1996 Dec;2(6):423-32. doi: 10.3109/13550289609146909. J Neurovirol. 1996. PMID: 8972425 - Brain Macrophages in Simian Immunodeficiency Virus-Infected, Antiretroviral-Suppressed Macaques: a Functional Latent Reservoir.
Avalos CR, Abreu CM, Queen SE, Li M, Price S, Shirk EN, Engle EL, Forsyth E, Bullock BT, Mac Gabhann F, Wietgrefe SW, Haase AT, Zink MC, Mankowski JL, Clements JE, Gama L. Avalos CR, et al. mBio. 2017 Aug 15;8(4):e01186-17. doi: 10.1128/mBio.01186-17. mBio. 2017. PMID: 28811349 Free PMC article. - Critical Role for Monocytes/Macrophages in Rapid Progression to AIDS in Pediatric Simian Immunodeficiency Virus-Infected Rhesus Macaques.
Sugimoto C, Merino KM, Hasegawa A, Wang X, Alvarez XA, Wakao H, Mori K, Kim WK, Veazey RS, Didier ES, Kuroda MJ. Sugimoto C, et al. J Virol. 2017 Aug 10;91(17):e00379-17. doi: 10.1128/JVI.00379-17. Print 2017 Sep 1. J Virol. 2017. PMID: 28566378 Free PMC article. - Monocyte/macrophage trafficking in acquired immunodeficiency syndrome encephalitis: lessons from human and nonhuman primate studies.
Fischer-Smith T, Bell C, Croul S, Lewis M, Rappaport J. Fischer-Smith T, et al. J Neurovirol. 2008 Aug;14(4):318-26. doi: 10.1080/13550280802132857. J Neurovirol. 2008. PMID: 18780233 Free PMC article. Review. - Brain macrophages harbor latent, infectious simian immunodeficiency virus.
Abreu C, Shirk EN, Queen SE, Beck SE, Mangus LM, Pate KAM, Mankowski JL, Gama L, Clements JE. Abreu C, et al. AIDS. 2019 Dec 1;33 Suppl 2(Suppl 2):S181-S188. doi: 10.1097/QAD.0000000000002269. AIDS. 2019. PMID: 31789817 Free PMC article. Review.
Cited by
- Quantitation of Productively Infected Monocytes and Macrophages of Simian Immunodeficiency Virus-Infected Macaques.
Avalos CR, Price SL, Forsyth ER, Pin JN, Shirk EN, Bullock BT, Queen SE, Li M, Gellerup D, O'Connor SL, Zink MC, Mankowski JL, Gama L, Clements JE. Avalos CR, et al. J Virol. 2016 May 27;90(12):5643-5656. doi: 10.1128/JVI.00290-16. Print 2016 Jun 15. J Virol. 2016. PMID: 27030272 Free PMC article. - Live attenuated simian immunodeficiency virus vaccination confers superinfection resistance against macrophage-tropic and neurovirulent wild-type SIV challenge.
Berry N, Ham C, Alden J, Clarke S, Stebbings R, Stott J, Ferguson D, Almond N. Berry N, et al. J Gen Virol. 2015 Jul;96(Pt 7):1918-29. doi: 10.1099/vir.0.000135. Epub 2015 Apr 1. J Gen Virol. 2015. PMID: 25834093 Free PMC article. - Important role of microglia in HIV-1 associated neurocognitive disorders and the molecular pathways implicated in its pathogenesis.
Borrajo A, Spuch C, Penedo MA, Olivares JM, Agís-Balboa RC. Borrajo A, et al. Ann Med. 2021 Dec;53(1):43-69. doi: 10.1080/07853890.2020.1814962. Epub 2020 Sep 17. Ann Med. 2021. PMID: 32841065 Free PMC article. Review. - Dopamine increases CD14+CD16+ monocyte migration and adhesion in the context of substance abuse and HIV neuropathogenesis.
Coley JS, Calderon TM, Gaskill PJ, Eugenin EA, Berman JW. Coley JS, et al. PLoS One. 2015 Feb 3;10(2):e0117450. doi: 10.1371/journal.pone.0117450. eCollection 2015. PLoS One. 2015. PMID: 25647501 Free PMC article. - Lipid nanoparticles for antisense oligonucleotide gene interference into brain border-associated macrophages.
Calero M, Moleiro LH, Sayd A, Dorca Y, Miquel-Rio L, Paz V, Robledo-Montaña J, Enciso E, Acción F, Herráez-Aguilar D, Hellweg T, Sánchez L, Bortolozzi A, Leza JC, García-Bueno B, Monroy F. Calero M, et al. Front Mol Biosci. 2022 Nov 3;9:887678. doi: 10.3389/fmolb.2022.887678. eCollection 2022. Front Mol Biosci. 2022. PMID: 36406277 Free PMC article.
References
- Barker C.F., Billingham R.E. Immunologically privileged sites. Adv. Immunol. 1977;25:1–6. - PubMed
- Balter M. AIDS ResearchHIV's other immune-system targets: macrophages. Science. 1996;274:1464–1465. - PubMed
- Cohen J. Exploring how to get at — and eradicate — hidden HIV. Science. 1998;279:1854–1855. - PubMed
- Dickson D.W., Mattiace L.A., Kure K., Hutchins K., Lyman W.D., Brosnan C.F. Microglia in human disease, with an emphasis on acquired immune deficiency syndrome. Lab. Invest. 1991;64:135–156. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS35732/NS/NINDS NIH HHS/United States
- R01 NS040237/NS/NINDS NIH HHS/United States
- NS40237/NS/NINDS NIH HHS/United States
- NS30769/NS/NINDS NIH HHS/United States
- R01 NS030769/NS/NINDS NIH HHS/United States
- R01 NS037654/NS/NINDS NIH HHS/United States
- NS37654/NS/NINDS NIH HHS/United States
- RR00168/RR/NCRR NIH HHS/United States
- P51 RR000168/RR/NCRR NIH HHS/United States
- K26 RR000168/RR/NCRR NIH HHS/United States