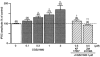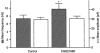Adenosine A(2A) receptor enhances GABA(A)-mediated IPSCs in the rat globus pallidus - PubMed (original) (raw)
Adenosine A(2A) receptor enhances GABA(A)-mediated IPSCs in the rat globus pallidus
T Shindou et al. J Physiol. 2001.
Abstract
1. The actions of adenosine A(2A) receptor agonists were examined on GABAergic synaptic transmission in the globus pallidus (GP) in rat brain slices using whole-cell patch-clamp recording. GP neurones were characterized into two major groups, type I and type II, according to the degree of time-dependent hyperpolarization-activated inward rectification and the size of input resistance. 2. The A(2A) receptor agonist 2-[p-(2-carboxyethyl)phenethylamino]-5'-N-ethylcarboxamido- adenosine (CGS21680; 0.3-3 microM) enhanced IPSCs evoked by stimulation within the GP. The actions of CGS21680 were blocked by the A(2A) antagonists (E)-8-(3,4-dimethoxystyryl)-1,3-dipropyl-7-methylxanthine (KF17837) and 4-(2-[7-amino-2-(2-furyl)[1,2,4]triazolo[2,3-a][1,3,5]triazin-5-ylamino]ethyl)phenol (ZM241385). 3. The CGS21680-induced increase in IPSCs was associated with a reduction in paired-pulse facilitation. CGS21680 (0.3 microM) increased the frequency of miniature IPSCs (mIPSCs) without affecting mIPSC amplitude. These observations demonstrated that the enhancement of IPSCs in the GP was attributable to presynaptic, but not postsynaptic, A(2A) receptors. 4. The results suggest that A(2A) receptors in the GP serve to inhibit GP neuronal activity, thereby disinhibiting subthalamic nucleus neurone activity. Thus, the A(2A) receptor-mediated presynaptic regulation in the GP, together with the A(2A) receptor-mediated intrastriatal presynaptic control of GABAergic neurotransmission described previously, may play a crucial role in controlling the neuronal functions of basal ganglia. This A(2A) receptor-mediated presynaptic dual control in the striatopallidal pathway could also afford the mode of action of A(2A) antagonists for ameliorating the symptoms of Parkinson's disease in an animal model.
Figures
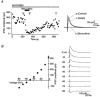
Figure 1. Visual and electrophysiological characterization of GP neurones
A, infrared photograph of a GP neurone in a rat slice preparation. B, micrograph of a biocytin-filled GP neurone that was visualized using histochemical procedures after being recorded in the whole-cell configuration. C, voltage responses and spontaneous discharges of type I neurone in the GP. Type I neurones showed little or slight sag during hyperpolarizing pulses (C1, average steady-state to peak sag ratio of 0.82 ± 0.03 in 8 neurones). Spontaneous action potentials fired at 9.6 Hz (C3). D, voltage responses and spontaneous discharges of type II neurone in the GP. Injection of a hyperpolarizing pulse produced a prominent sag in the membrane potential in type II neurones (D1, average steady-state to peak sag ratio of 0.66 ± 0.01 in 16 neurones, P < 0.005 vs. type I neurones by Wilcoxon's rank sum test). Both type of neurones fired regular spikes from hyperpolarized potentials (C2 and D2). Voltage, time and current calibrations in C1 also apply to C2, D1 and D2. Scale bars: A, 20 μm; B, 100 μm.
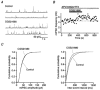
Figure 2. Evoked GABAergic postsynaptic currents in GP neurones
Evoked IPSCs were induced by focal stimulation within the GP in GP neurones in a solution containing antagonists for non-NMDA and NMDA receptors (10 μ
m
CNQX and 50 μ
m
APV). A, time course of the amplitude of evoked IPSCs during the application of GABAA receptor antagonist (10 μ
m
bicuculline) (left), and superimposed traces (right) of an average of consecutive evoked IPSCs (5 traces) before (control) and during application of bicuculline and after (wash), taken at the indicated time points. Bicuculline was applied to the superfusion medium for the period indicated by the bar. Membrane potential = 0 mV. B, reversal potentials of GABAergic IPSCs. It reversed polarity at -50 mV, close to the chloride equilibrium potential.
Figure 3. CGS21680-induced enhancement of evoked IPSCs in the GP
A, enhancement of evoked IPSCs by an adenosine A2A receptor agonist, CGS21680. Left, the time course of the amplitude of evoked IPSCs during the application of CGS21680 (1 μ
m
); right, superimposed traces of an average of consecutive evoked IPSCs (8 traces) before (control) and during application of CGS21680 (1 μ
m
), taken at the indicated time points. B, effect of the adenosine A2A receptor antagonist KF17837 (0.5 μ
m
) on the CGS21680-induced enhancement of evoked IPSCs. KF17837 was applied to the superfusion medium for the period indicated by the bar. Evoked IPSCs were recorded in the presence of CNQX (10 μ
m
) and APV (50 μ
m
) at a holding potential of 0 mV.
Figure 4. Summary of pharmacological characterization of the adenosine A2A receptor-mediated GABAergic synaptic transmission
Data are normalized as a percentage of control values. The error bars represent
s.e.m.
The numbers of cells examined are given in parentheses. *P < 0.05 vs. 0 μ
m
CGS21680 by Steel's test; †P < 0.05, ††P < 0.005 vs. 1 μ
m
CGS21680 by Wilcoxon's rank sum test.
Figure 5. Effects of CGS21680 on PPF
A, reduction of PPF by CGS21680. The upper traces show typical superimposed traces of an average of consecutive IPSCs (8 traces) evoked by paired stimulation (50 ms interval) before (control) and during application of CGS21680 (1 μ
m
). The lower traces are the same as those above, except that the amplitude of the first IPSC recorded in control conditions has been normalized to the first IPSC recorded during CGS21680 application. B, mean PPF ratios in 8 different neurones under control conditions and in the presence of CGS21680 (1 μ
m
). PPF is decreased in the presence of CGS21680. The error bars represent
s.e.m.
*P < 0.001 vs. control by paired t test.
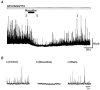
Figure 6. Spontaneous miniature GABAergic IPSCs in GP neurones
Spontaneous mIPSCs were recorded in a solution containing CNQX (10 μ
m
), APV (50 μ
m
) and TTX (0.5 μm). A, a continuous trace showing time course of mIPSCs during application of GABAA receptor antagonist (10 μ
m
bicuculline). B, example traces with an expanded time scale from A, taken at the indicated time points. These mIPSCs were recorded at a holding potential of 0 mV.
Figure 7. Analysis of spontaneous miniature IPSCs in the GP neurones
The data are taken from the same neurone. A, two consecutive traces of mIPSCs before (control) and during application of CGS21680 (0.3 μ
m
). B, time course of the frequency of mIPSCs during application of CGS21680 (0.3 μ
m
). C, cumulative probability distribution of mIPSC amplitude (left) and inter-event interval (right) before (1255 events) and during application of CGS21680 (1802 events). CGS21680 had no effect on the amplitude distribution (P > 0.2, Kolmogorov-Smirnov test for control vs. CGS21680), but shifted the frequency distribution to shorter inter-event intervals (P < 0.001, Kolmogorov-Smirnov test for control vs. CGS21680). The frequencies and amplitudes (mean ±
s.d.
) of mIPSCs were 4.59 Hz and 38.75 ± 24.08 pA in control and 6.01 Hz and 37.72 ± 23.14 pA during CGS21680 application, respectively.
Figure 8. Summary graph of the experiments which tested the effect of CGS21680 on mIPSC frequency and amplitude
Pooled data of 9 neurones show that CGS21680 (0.3 μ
m
) increased the mean frequency without affecting the mean amplitude of mIPSCs. Mean frequency ( ) and mean amplitude (□) of mIPSC were 7.4 ± 0.9 Hz and 29.76 ± 2.36 pA before (control) and 9.8 ± 1.4 Hz and 30.75 ± 2.39 pA during application of CGS21680, respectively. CGS21680 increased the frequency of mIPSCs by 130 ± 4 %. The error bars represent
) and mean amplitude (□) of mIPSC were 7.4 ± 0.9 Hz and 29.76 ± 2.36 pA before (control) and 9.8 ± 1.4 Hz and 30.75 ± 2.39 pA during application of CGS21680, respectively. CGS21680 increased the frequency of mIPSCs by 130 ± 4 %. The error bars represent
s.e.m.
*P < 0.01 vs. control by paired t test.
Comment in
- The adenosine A(2A) receptor of the basal ganglia.
Richardson PJ. Richardson PJ. J Physiol. 2001 Apr 15;532(Pt 2):284. doi: 10.1111/j.1469-7793.2001.0284f.x. J Physiol. 2001. PMID: 11306648 Free PMC article. No abstract available.
Similar articles
- Presynaptic adenosine A2A receptors enhance GABAergic synaptic transmission via a cyclic AMP dependent mechanism in the rat globus pallidus.
Shindou T, Nonaka H, Richardson PJ, Mori A, Kase H, Ichimura M. Shindou T, et al. Br J Pharmacol. 2002 May;136(2):296-302. doi: 10.1038/sj.bjp.0704702. Br J Pharmacol. 2002. PMID: 12010779 Free PMC article. - Synaptically released GABA activates both pre- and postsynaptic GABA(B) receptors in the rat globus pallidus.
Kaneda K, Kita H. Kaneda K, et al. J Neurophysiol. 2005 Aug;94(2):1104-14. doi: 10.1152/jn.00255.2005. J Neurophysiol. 2005. PMID: 16061489 - Presynaptic mu and delta opioid receptor modulation of GABAA IPSCs in the rat globus pallidus in vitro.
Stanford IM, Cooper AJ. Stanford IM, et al. J Neurosci. 1999 Jun 15;19(12):4796-803. doi: 10.1523/JNEUROSCI.19-12-04796.1999. J Neurosci. 1999. PMID: 10366614 Free PMC article. - The role of the adenosinergic system in lung fibrosis.
Della Latta V, Cabiati M, Rocchiccioli S, Del Ry S, Morales MA. Della Latta V, et al. Pharmacol Res. 2013 Oct;76:182-9. doi: 10.1016/j.phrs.2013.08.004. Epub 2013 Aug 28. Pharmacol Res. 2013. PMID: 23994158 Review. - Anatomy of adenosine A2A receptors in brain: morphological substrates for integration of striatal function.
Rosin DL, Hettinger BD, Lee A, Linden J. Rosin DL, et al. Neurology. 2003 Dec 9;61(11 Suppl 6):S12-8. doi: 10.1212/01.wnl.0000095205.33940.99. Neurology. 2003. PMID: 14663003 Review.
Cited by
- Dopamine D4 receptor-induced postsynaptic inhibition of GABAergic currents in mouse globus pallidus neurons.
Shin RM, Masuda M, Miura M, Sano H, Shirasawa T, Song WJ, Kobayashi K, Aosaki T. Shin RM, et al. J Neurosci. 2003 Dec 17;23(37):11662-72. doi: 10.1523/JNEUROSCI.23-37-11662.2003. J Neurosci. 2003. PMID: 14684868 Free PMC article. - Role of adenosine A2A receptors in motor control: relevance to Parkinson's disease and dyskinesia.
Pinna A, Serra M, Morelli M, Simola N. Pinna A, et al. J Neural Transm (Vienna). 2018 Aug;125(8):1273-1286. doi: 10.1007/s00702-018-1848-6. Epub 2018 Feb 2. J Neural Transm (Vienna). 2018. PMID: 29396609 Review. - Adenosine A2A receptors and basal ganglia physiology.
Schiffmann SN, Fisone G, Moresco R, Cunha RA, Ferré S. Schiffmann SN, et al. Prog Neurobiol. 2007 Dec;83(5):277-92. doi: 10.1016/j.pneurobio.2007.05.001. Epub 2007 Jun 26. Prog Neurobiol. 2007. PMID: 17646043 Free PMC article. Review. - Presynaptic adenosine A2A receptors enhance GABAergic synaptic transmission via a cyclic AMP dependent mechanism in the rat globus pallidus.
Shindou T, Nonaka H, Richardson PJ, Mori A, Kase H, Ichimura M. Shindou T, et al. Br J Pharmacol. 2002 May;136(2):296-302. doi: 10.1038/sj.bjp.0704702. Br J Pharmacol. 2002. PMID: 12010779 Free PMC article. - Potential therapeutic interest of adenosine A2A receptors in psychiatric disorders.
Cunha RA, Ferré S, Vaugeois JM, Chen JF. Cunha RA, et al. Curr Pharm Des. 2008;14(15):1512-24. doi: 10.2174/138161208784480090. Curr Pharm Des. 2008. PMID: 18537674 Free PMC article. Review.
References
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends in Neurosciences. 1989;12:366–375. - PubMed
- Augood SJ, Emson PC. Adenosine A2a receptor mRNA is expressed by enkephalin cells but not by somatostatin cells in rat striatum: a co-expression study. Molecular Brain Research. 1994;22:204–210. - PubMed
- Chesselet MF, Delfs JM. Basal ganglia and movement disorders: an update. Trends in Neurosciences. 1996;19:417–422. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources