Integrin-mediated adhesion regulates ERK nuclear translocation and phosphorylation of Elk-1 - PubMed (original) (raw)
Integrin-mediated adhesion regulates ERK nuclear translocation and phosphorylation of Elk-1
A E Aplin et al. J Cell Biol. 2001.
Abstract
Integrin-mediated adhesion to the extracellular matrix permits efficient growth factor-mediated activation of extracellular signal-regulated kinases (ERKs). Points of regulation have been localized to the level of receptor phosphorylation or to activation of the downstream components, Raf and MEK (mitogen-activated protein kinase/ERK kinase). However, it is also well established that ERK translocation from the cytoplasm to the nucleus is required for G1 phase cell cycle progression. Here we show that phosphorylation of the nuclear ERK substrate, Elk-1 at serine 383, is anchorage dependent in response to growth factor treatment of NIH 3T3 fibroblasts. Furthermore, when we activated ERK in nonadherent cells by expression of active components of the ERK cascade, subsequent phosphorylation of Elk-1 at serine 383 and Elk-1-mediated transactivation were still impaired compared with adherent cells. Elk-1 phosphorylation was dependent on an intact actin cytoskeleton, as discerned by treatment with cytochalasin D (CCD). Finally, expression of active MEK failed to predominantly localize ERK to the nucleus in suspended cells or adherent cells treated with CCD. These data show that integrin-mediated organization of the actin cytoskeleton regulates localization of activated ERK, and in turn the ability of ERK to efficiently phosphorylate nuclear substrates.
Figures
Figure 1
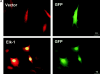
Adhesion to fibronectin and EGF collaborate to provide efficient phosphorylation of the Elk-1 transcription factor. NIH 3T3 cells were transfected with either pCMV5 (Vector) or pCMV5-FLAG-Elk-1. In A, 1 μg of green fluorescent protein (GFP) was included in the transfections to identify transfected cells. After 48 h, transfected cells were serum starved before being replated in DMEM/BSA on fibronectin-coated coverslips (A) or maintained in suspension (Sus) or replated on fibronectin (Fn)-coated dishes for a further 3 h (B). (A) Localization of Elk-1 was determined by immunofluorescence with an Elk-1 antibody and TRITC-conjugated anti–rabbit secondary antibody. The scale bar depicts a 10 micron distance. (B) After the 3-h incubation, cells were treated with 20 ng/ml EGF for 15 min as indicated. Ectopically expressed Elk-1 was immunoprecipitated (IP) from cell lysates from each condition with an M2 FLAG epitope antibody. Immunoprecipitates were analyzed by Western blotting (WB) with antibodies to determine phosphorylated and total Elk-1 levels.
Figure 1
Adhesion to fibronectin and EGF collaborate to provide efficient phosphorylation of the Elk-1 transcription factor. NIH 3T3 cells were transfected with either pCMV5 (Vector) or pCMV5-FLAG-Elk-1. In A, 1 μg of green fluorescent protein (GFP) was included in the transfections to identify transfected cells. After 48 h, transfected cells were serum starved before being replated in DMEM/BSA on fibronectin-coated coverslips (A) or maintained in suspension (Sus) or replated on fibronectin (Fn)-coated dishes for a further 3 h (B). (A) Localization of Elk-1 was determined by immunofluorescence with an Elk-1 antibody and TRITC-conjugated anti–rabbit secondary antibody. The scale bar depicts a 10 micron distance. (B) After the 3-h incubation, cells were treated with 20 ng/ml EGF for 15 min as indicated. Ectopically expressed Elk-1 was immunoprecipitated (IP) from cell lysates from each condition with an M2 FLAG epitope antibody. Immunoprecipitates were analyzed by Western blotting (WB) with antibodies to determine phosphorylated and total Elk-1 levels.
Figure 3
Phosphorylation and transcriptional activity of Elk-1 mediated by activated ERK are impaired in nonadherent cells. NIH 3T3 cells were transfected with FLAG–Elk-1 and either vector (Vec), 22W Raf (A), or MEK1-ΔED (B). Serum-starved cells were either replated on fibronectin-coated plates (Fn) or maintained in suspension (Sus) for 3 h. FLAG-Elk-1 immunoprecipitates (IP) were analyzed by Western blotting (WB) for levels of serine 383 phosphorylated and total Elk-1. Shown are representatives of at least three independent experiments with equivalent results. In C, cells were transfected with GAL4–Elk-1, pFR-luc reporter, and either vector or 22W Raf. After a brief serum starvation, cells were replated as above on fibronectin-coated plates (Fn) or maintained in suspension (Sus). The increase in GAL4-Elk-1 transactivation of pFR-luc during a 4-h time period was determined by assaying for firefly luciferase activity. For each experiment, three separate samples were assayed for each condition and all readings were normalized to the activity of Renilla luciferase under the control of a constitutively active CMV promoter (pRL-CMV-luc). The enhanced GAL4–Elk-1–driven luciferase activity in 22W Raf–expressing cells in adherent compared with suspended cells is statistically significant (*P < 0.05).
Figure 3
Phosphorylation and transcriptional activity of Elk-1 mediated by activated ERK are impaired in nonadherent cells. NIH 3T3 cells were transfected with FLAG–Elk-1 and either vector (Vec), 22W Raf (A), or MEK1-ΔED (B). Serum-starved cells were either replated on fibronectin-coated plates (Fn) or maintained in suspension (Sus) for 3 h. FLAG-Elk-1 immunoprecipitates (IP) were analyzed by Western blotting (WB) for levels of serine 383 phosphorylated and total Elk-1. Shown are representatives of at least three independent experiments with equivalent results. In C, cells were transfected with GAL4–Elk-1, pFR-luc reporter, and either vector or 22W Raf. After a brief serum starvation, cells were replated as above on fibronectin-coated plates (Fn) or maintained in suspension (Sus). The increase in GAL4-Elk-1 transactivation of pFR-luc during a 4-h time period was determined by assaying for firefly luciferase activity. For each experiment, three separate samples were assayed for each condition and all readings were normalized to the activity of Renilla luciferase under the control of a constitutively active CMV promoter (pRL-CMV-luc). The enhanced GAL4–Elk-1–driven luciferase activity in 22W Raf–expressing cells in adherent compared with suspended cells is statistically significant (*P < 0.05).
Figure 3
Phosphorylation and transcriptional activity of Elk-1 mediated by activated ERK are impaired in nonadherent cells. NIH 3T3 cells were transfected with FLAG–Elk-1 and either vector (Vec), 22W Raf (A), or MEK1-ΔED (B). Serum-starved cells were either replated on fibronectin-coated plates (Fn) or maintained in suspension (Sus) for 3 h. FLAG-Elk-1 immunoprecipitates (IP) were analyzed by Western blotting (WB) for levels of serine 383 phosphorylated and total Elk-1. Shown are representatives of at least three independent experiments with equivalent results. In C, cells were transfected with GAL4–Elk-1, pFR-luc reporter, and either vector or 22W Raf. After a brief serum starvation, cells were replated as above on fibronectin-coated plates (Fn) or maintained in suspension (Sus). The increase in GAL4-Elk-1 transactivation of pFR-luc during a 4-h time period was determined by assaying for firefly luciferase activity. For each experiment, three separate samples were assayed for each condition and all readings were normalized to the activity of Renilla luciferase under the control of a constitutively active CMV promoter (pRL-CMV-luc). The enhanced GAL4–Elk-1–driven luciferase activity in 22W Raf–expressing cells in adherent compared with suspended cells is statistically significant (*P < 0.05).
Figure 2
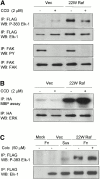
Expression of active Raf or MEK in suspended cells is sufficient to activate ERK activity. (A) NIH 3T3 cells transfected either with pcDNA3 vector (Vec) or pcDNA3-22W Raf were analyzed by Western blotting (WB) with the anti-Raf COOH-terminal (C-term) antibody, C12, 48 h after transfection. (B) Cells transfected with HA-ERK1 and either vector (Vec) or 22W Raf were replated onto fibronectin (Fn) or maintained in suspension (Sus) for 3 h, as above. Some cells were then treated with 10 ng/ml EGF for 5 min as indicated. HA-ERK was immunoprecipitated (IP) from cell lysates and assayed for activity by in vitro kinase assay using myelin basic protein (MBP) as a substrate. (C) HA-ERK activity was measured in vector and 22W Raf–expressing cells either under nonadherent or adherent conditions at several time points. Values were normalized to the activity of HA-ERK at the 2 h time point on Fn. Shown is the mean and standard deviation of three independent experiments. (D) Cells were cotransfected with equivalent levels of HA-ERK1 and MEK1-ΔED. HA-ERK activity was analyzed, as above, in cells maintained in DMEM/BSA in suspension and adherent conditions for 3 h.
Figure 4
Disruption of the actin cytoskeleton, but not the microtubule network, inhibits the ability of activated ERK to phosphorylate Elk-1. NIH 3T3 cells were transfected either with vector (Vec) or 22W Raf and either FLAG-Elk-1 (A and C) or HA-ERK1 (B). In A and B, cells were treated accordingly with 2 μM CCD throughout adhesion to fibronectin-coated plates (Fn). In C, serum-starved cells were treated with 50 μM colchicine (Colc), as indicated, before replating either on fibronectin-coated plates (Fn) or maintained in suspension (Sus) for 3 h. FLAG-Elk-1 immunoprecipitates (IP) were analyzed by Western blotting (WB) for levels of serine 383 phosphorylated and total Elk-1 (A and C). Additionally, in A (bottom) endogenous FAK was immunoprecipitated from cell lysates and blotted for tyrosine phosphorylation (PY) and total levels of FAK. In B, HA-ERK was immunoprecipitated and activity measured by in vitro kinase assay. Shown are representatives of at least three independent experiments with equivalent results.
Figure 5
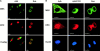
Nuclear accumulation of ERK is impaired in nonadherent cells and by disruption of the actin cytoskeleton. Tet-Mek*-3T3 cells were serum starved and stimulated with 10% FCS in the absence of tetracycline for 6–9 h. The localization of active MEK and ERK was compared in adherent (Adh) and nonadherent (Sus) cells or adherent untreated (Adh) vs. CCD-treated (Adh/CCD) monolayers via confocal microscopy. Bottom panels are of (A) overlays of the MEK and ERK images or (B) images showing DAPI staining of nuclei. Scale bars represent either a 10 or 5 micron distance, as indicated. (C and D) tet-MEK*-3T3 cells were transfected with FLAG–Elk-1 as before. Cells were serum starved overnight, after which in some populations tetracycline was removed from the media to induce expression of active MEK. Cells were detached and either maintained in suspension (Sus) or replated onto fibronectin (Fn) and lysed 6 h after induction. (C) Levels of MEK expression and activation of ERK determined by Western blotting (WB) of whole cell lysates. (D) FLAG–Elk-1 was immunoprecipitated (IP) and analyzed by Western blotting for phosphorylated and total levels of Elk-1.
Figure 5
Nuclear accumulation of ERK is impaired in nonadherent cells and by disruption of the actin cytoskeleton. Tet-Mek*-3T3 cells were serum starved and stimulated with 10% FCS in the absence of tetracycline for 6–9 h. The localization of active MEK and ERK was compared in adherent (Adh) and nonadherent (Sus) cells or adherent untreated (Adh) vs. CCD-treated (Adh/CCD) monolayers via confocal microscopy. Bottom panels are of (A) overlays of the MEK and ERK images or (B) images showing DAPI staining of nuclei. Scale bars represent either a 10 or 5 micron distance, as indicated. (C and D) tet-MEK*-3T3 cells were transfected with FLAG–Elk-1 as before. Cells were serum starved overnight, after which in some populations tetracycline was removed from the media to induce expression of active MEK. Cells were detached and either maintained in suspension (Sus) or replated onto fibronectin (Fn) and lysed 6 h after induction. (C) Levels of MEK expression and activation of ERK determined by Western blotting (WB) of whole cell lysates. (D) FLAG–Elk-1 was immunoprecipitated (IP) and analyzed by Western blotting for phosphorylated and total levels of Elk-1.
Figure 6
Targeting of cyclin D1 to the nucleus in not affected by disruption of the actin cytoskeleton. Tet-cyclin D1-3T3 cells were G0-synchronized and stimulated with 10% FCS in the absence of tetracycline in monolayer in the absence and presence of CCD. The cells were fixed 6 h after stimulation, stained with anti-cyclin D1 antibody and DAPI nuclear stain, and analyzed via confocal microscopy. An overlay of the cyclin D1 and DAPI images is shown. The scale bars show a 10 micron distance. Adh, adherent.
Similar articles
- Cell adhesion differentially regulates the nucleocytoplasmic distribution of active MAP kinases.
Aplin AE, Hogan BP, Tomeu J, Juliano RL. Aplin AE, et al. J Cell Sci. 2002 Jul 1;115(Pt 13):2781-90. doi: 10.1242/jcs.115.13.2781. J Cell Sci. 2002. PMID: 12077368 - Growth hormone stimulates phosphorylation and activation of elk-1 and expression of c-fos, egr-1, and junB through activation of extracellular signal-regulated kinases 1 and 2.
Hodge C, Liao J, Stofega M, Guan K, Carter-Su C, Schwartz J. Hodge C, et al. J Biol Chem. 1998 Nov 20;273(47):31327-36. doi: 10.1074/jbc.273.47.31327. J Biol Chem. 1998. PMID: 9813041 - Lack of Elk-1 phosphorylation and dysregulation of the extracellular regulated kinase signaling pathway in senescent human fibroblast.
Tresini M, Lorenzini A, Frisoni L, Allen RG, Cristofalo VJ. Tresini M, et al. Exp Cell Res. 2001 Oct 1;269(2):287-300. doi: 10.1006/excr.2001.5334. Exp Cell Res. 2001. PMID: 11570821 - Phosphorylation of hippocampal Erk-1/2, Elk-1, and p90-Rsk-1 during contextual fear conditioning: interactions between Erk-1/2 and Elk-1.
Sananbenesi F, Fischer A, Schrick C, Spiess J, Radulovic J. Sananbenesi F, et al. Mol Cell Neurosci. 2002 Nov;21(3):463-76. doi: 10.1006/mcne.2002.1188. Mol Cell Neurosci. 2002. PMID: 12498787
Cited by
- Defined extracellular matrix compositions support stiffness-insensitive cell spreading and adhesion signaling.
Conway JRW, Isomursu A, Follain G, Härmä V, Jou-Ollé E, Pasquier N, Välimäki EPO, Rantala JK, Ivaska J. Conway JRW, et al. Proc Natl Acad Sci U S A. 2023 Oct 24;120(43):e2304288120. doi: 10.1073/pnas.2304288120. Epub 2023 Oct 16. Proc Natl Acad Sci U S A. 2023. PMID: 37844244 Free PMC article. - Specificity models in MAPK cascade signaling.
Ma Y, Nicolet J. Ma Y, et al. FEBS Open Bio. 2023 Jul;13(7):1177-1192. doi: 10.1002/2211-5463.13619. Epub 2023 Jun 11. FEBS Open Bio. 2023. PMID: 37157227 Free PMC article. Review. - Neurodevelopmental disorders, like cancer, are connected to impaired chromatin remodelers, PI3K/mTOR, and PAK1-regulated MAPK.
Nussinov R, Yavuz BR, Arici MK, Demirel HC, Zhang M, Liu Y, Tsai CJ, Jang H, Tuncbag N. Nussinov R, et al. Biophys Rev. 2023 Apr 1;15(2):163-181. doi: 10.1007/s12551-023-01054-9. eCollection 2023 Apr. Biophys Rev. 2023. PMID: 37124926 Free PMC article. Review. - Development of a novel cell-based, In-Cell Western/ERK assay system for the high-throughput screening of agonists acting on the delta-opioid receptor.
Asghar J, Latif L, Alexander SPH, Kendall DA. Asghar J, et al. Front Pharmacol. 2022 Sep 26;13:933356. doi: 10.3389/fphar.2022.933356. eCollection 2022. Front Pharmacol. 2022. PMID: 36225576 Free PMC article. - Substratum stiffness regulates Erk signaling dynamics through receptor-level control.
Farahani PE, Lemke SB, Dine E, Uribe G, Toettcher JE, Nelson CM. Farahani PE, et al. Cell Rep. 2021 Dec 28;37(13):110181. doi: 10.1016/j.celrep.2021.110181. Cell Rep. 2021. PMID: 34965432 Free PMC article.
References
- Albanese C., Johnson J., Watanabe G., Eklund N., Vu D., Arnold A., Pestell R.G. Transforming p21ras mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. J. Biol. Chem. 1995;270:23589–23597. - PubMed
- Aplin A.E., Juliano R.L. Integrin and cytoskeletal regulation of growth factor signaling to the MAP kinase pathway. J. Cell Sci. 1999;112:695–706. - PubMed
- Aplin A.E., Short S.M., Juliano R.L. Anchorage-dependent regulation of the mitogen-activated protein kinase cascade by growth factors is supported by a variety of integrin alpha chains. J. Biol. Chem. 1999;274:31223–31228. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous