Cvt9/Gsa9 functions in sequestering selective cytosolic cargo destined for the vacuole - PubMed (original) (raw)
Cvt9/Gsa9 functions in sequestering selective cytosolic cargo destined for the vacuole
J Kim et al. J Cell Biol. 2001.
Abstract
Three overlapping pathways mediate the transport of cytoplasmic material to the vacuole in Saccharomyces cerevisiae. The cytoplasm to vacuole targeting (Cvt) pathway transports the vacuolar hydrolase, aminopeptidase I (API), whereas pexophagy mediates the delivery of excess peroxisomes for degradation. Both the Cvt and pexophagy pathways are selective processes that specifically recognize their cargo. In contrast, macroautophagy nonselectively transports bulk cytosol to the vacuole for recycling. Most of the import machinery characterized thus far is required for all three modes of transport. However, unique features of each pathway dictate the requirement for additional components that differentiate these pathways from one another, including at the step of specific cargo selection.We have identified Cvt9 and its Pichia pastoris counterpart Gsa9. In S. cerevisiae, Cvt9 is required for the selective delivery of precursor API (prAPI) to the vacuole by the Cvt pathway and the targeted degradation of peroxisomes by pexophagy. In P. pastoris, Gsa9 is required for glucose-induced pexophagy. Significantly, neither Cvt9 nor Gsa9 is required for starvation-induced nonselective transport of bulk cytoplasmic cargo by macroautophagy. The deletion of CVT9 destabilizes the binding of prAPI to the membrane and analysis of a cvt9 temperature-sensitive mutant supports a direct role of Cvt9 in transport vesicle formation. Cvt9 oligomers peripherally associate with a novel, perivacuolar membrane compartment and interact with Apg1, a Ser/Thr kinase essential for both the Cvt pathway and autophagy. In P. pastoris Gsa9 is recruited to concentrated regions on the vacuole membrane that contact peroxisomes in the process of being engulfed by pexophagy. These biochemical and morphological results demonstrate that Cvt9 and the P. pastoris homologue Gsa9 may function at the step of selective cargo sequestration.
Figures
Figure 1
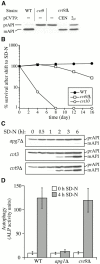
Cvt9 is essential for the Cvt pathway but not for autophagy. (A) CVT9 complements the prAPI transport defect in the cvt9 mutants. Wild-type (WT; SEY6210), cvt9 (AHY96), and _cvt9_Δ (AHY001) strains transformed with either the centromeric (CEN) or multicopy (2μ) CVT9 plasmid (pCVT9) were grown to midlog phase in SMD. Protein extracts were prepared and analyzed by immunoblots using antiserum to API. The positions of precursor and mature API are indicated. (B) The _cvt9_Δ strain is largely resistant to nitrogen starvation conditions. Wild-type (SEY6210), _cvt9_Δ (AHY001), and cvt10/apg1 (AHY1468) cells were grown to midlog phase in SMD and transferred to SD-N as described in Materials and Methods. Aliquots were removed at the indicated times and spread onto YPD plates in triplicate. Numbers of viable colonies were determined after 2–3 d. (C) The accumulation of prAPI in _cvt9_Δ can be partially reversed by nitrogen starvation. The _apg7_Δ (VDY101), cvt3 (THY119), and _cvt9_Δ (AHY001) strains were grown to midlog phase and shifted to SD-N. Protein extracts were prepared at the indicated times and analyzed by immunoblots with antiserum to API. (D) ALP activity assay for autophagy. Wild-type (TN125), _apg1_Δ (YYK126), and _cvt9_Δ (YYK127) cells expressing Pho8Δ60 were shifted from nutrient-rich medium (white bars) to SD-N nitrogen-depleted medium (black bars) for 4 h. The level of autophagy induction was determined by an ALP activity assay as described in Materials and Methods. The graph is the average of three experiments. The _cvt9_Δ strain is not defective in the vacuolar delivery of the autophagy marker Pho8Δ60 during starvation conditions. (E) Cvt9 is not required for the formation of autophagosomes. The _cvt9_Δ (YYK127) strain was grown in nutrient-rich (YPD) and nitrogen starvation (SD-N) conditions supplemented with the protease inhibitor PMSF and examined by electron microscopy as described in Materials and Methods. In YPD, Cvt complexes (indicated by an arrow) could be detected in the cytoplasm. In SD-N, _cvt9_Δ cells accumulated autophagic bodies in the vacuole when treated with PMSF.
Figure 1
Cvt9 is essential for the Cvt pathway but not for autophagy. (A) CVT9 complements the prAPI transport defect in the cvt9 mutants. Wild-type (WT; SEY6210), cvt9 (AHY96), and _cvt9_Δ (AHY001) strains transformed with either the centromeric (CEN) or multicopy (2μ) CVT9 plasmid (pCVT9) were grown to midlog phase in SMD. Protein extracts were prepared and analyzed by immunoblots using antiserum to API. The positions of precursor and mature API are indicated. (B) The _cvt9_Δ strain is largely resistant to nitrogen starvation conditions. Wild-type (SEY6210), _cvt9_Δ (AHY001), and cvt10/apg1 (AHY1468) cells were grown to midlog phase in SMD and transferred to SD-N as described in Materials and Methods. Aliquots were removed at the indicated times and spread onto YPD plates in triplicate. Numbers of viable colonies were determined after 2–3 d. (C) The accumulation of prAPI in _cvt9_Δ can be partially reversed by nitrogen starvation. The _apg7_Δ (VDY101), cvt3 (THY119), and _cvt9_Δ (AHY001) strains were grown to midlog phase and shifted to SD-N. Protein extracts were prepared at the indicated times and analyzed by immunoblots with antiserum to API. (D) ALP activity assay for autophagy. Wild-type (TN125), _apg1_Δ (YYK126), and _cvt9_Δ (YYK127) cells expressing Pho8Δ60 were shifted from nutrient-rich medium (white bars) to SD-N nitrogen-depleted medium (black bars) for 4 h. The level of autophagy induction was determined by an ALP activity assay as described in Materials and Methods. The graph is the average of three experiments. The _cvt9_Δ strain is not defective in the vacuolar delivery of the autophagy marker Pho8Δ60 during starvation conditions. (E) Cvt9 is not required for the formation of autophagosomes. The _cvt9_Δ (YYK127) strain was grown in nutrient-rich (YPD) and nitrogen starvation (SD-N) conditions supplemented with the protease inhibitor PMSF and examined by electron microscopy as described in Materials and Methods. In YPD, Cvt complexes (indicated by an arrow) could be detected in the cytoplasm. In SD-N, _cvt9_Δ cells accumulated autophagic bodies in the vacuole when treated with PMSF.
Figure 2
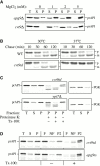
CVT9 mutations destabilize prAPI membrane binding and cause a defect in vesicle formation. (A) prAPI membrane binding is destabilized in the _cvt9_Δ mutant. The _cvt9_Δ (AHY001) and _apg9_Δ (JKY007) strains were grown to midlog phase and converted to spheroplasts. The spheroplasts were lysed in osmotic lysis buffer supplemented with 0, 1, 2, or 5 mM MgCl2. The total lysate (T) was separated into low speed supernatant (S) and pellet (P) fractions by centrifugation at 2,300 g for 5 min. The fractionated samples were subjected to immunoblot analysis using antiserum to API. (B) The temperature-conditional cvt9td strain is tightly blocked for prAPI import at nonpermissive temperature. Wild-type (WT; SEY6210) and cvt9td (JGY9td) strains were incubated at 30°C and 37°C for 5 min, pulse-labeled for 10 min, and then subjected to nonradioactive chase reactions for the indicated times. Samples at each time point were immunoprecipitated with antiserum to API as described in Materials and Methods. (C) prAPI in the cvt9td strain is protease accessible. Spheroplasts isolated from cvt9td and _ypt7_Δ cells were pulse-labeled for 10 min and chased for 30 min at 37°C. The labeled spheroplasts were then osmotically lysed and separated into low-speed supernatant (S) and pellet (P) fractions after a 2,300-g centrifugation step. The pellet fractions were subjected to protease treatment in the absence or presence of 0.2% Triton X-100 as described in Materials and Methods. (D) prAPI in the cvt9td strain associates with a floatable membrane fraction. Spheroplasts of apg9ts and cvt9td were osmotically lysed and separated into supernatant (S) and pellet (P) fractions by centrifugation at 2,300 g for 5 min. An aliquot was removed for the total lysate control (T). The pellet fraction (P) was resuspended in 15% Ficoll-400 and added to the bottom of a step gradient of 13 and 2% Ficoll-400. The step gradients were centrifuged at 16,000 g for 10 min. Membrane-containing float (F), nonfloat (NF), and pellet (P2) fractions were immunoprecipitated with antiserum to API as described in Materials and Methods.
Figure 3
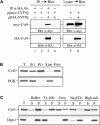
Characterization of Cvt9. (A) Cvt9 forms a homodimer. The wild-type (KA311A) strain was transformed with a plasmid encoding myc-tagged Cvt9 with or without a second plasmid for HA-tagged Cvt9. Cell lysates were prepared as described in Materials and Methods and immunoprecipitated (IP) with antiserum to HA (lanes 1 and 3) or buffer control (lanes 2 and 4). The immunoprecipitates were then immunoblotted with antiserum or antibodies to HA and myc. The anti-HA–precipitated immunocomplex also contains myc-tagged Cvt9. The cell lysates were also directly immunoblotted with anti-HA and anti-myc antibodies to assess the expression of the epitope tagged Cvt9 fusions (lanes 5 and 6). (B) Cvt9 subcellular fractionation pattern. The _cvt9_Δ (AHY001) strain was transformed with a CVT9 plasmid behind the CUP1 copper-inducible promoter (pCuCVT9[416]). Expression was induced with 50 μM CuSO4 for 1 h before spheroplasting. The spheroplasts were osmotically lysed (see Materials and Methods). After a preclearing centrifugation step at 100 g for 5 min to remove unlysed spheroplasts, the total lysate (T) was separated into low speed supernatant (S13) and pellet (P13) fractions. The S13 fraction was further separated into 100,000 g supernatant (S100) and pellet (P100) fractions. The fractionated samples were subjected to immunoblot analysis using antiserum to Cvt9 and the cytosolic marker protein PGK. (C) Cvt9 is a peripheral membrane protein that is detergent resistant. A total lysate was prepared as in B and resuspended in osmotic lysis buffer only or containing 1% Triton X-100, 6 M urea, 0.1 M Na2CO3, pH 10.5, or a 1.0 M salt wash (0.67 M KOAc, 0.3 M KCl). The treated lysates were separated into total membrane (P) and supernatant (S) fractions by centrifugation at 100,000 g for 20 min at 4°C. The samples were analyzed by immunoblots with antiserum to Cvt9 and antibodies to the ER integral membrane protein Dpm1.
Figure 4
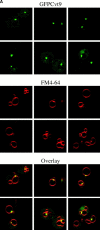
Cvt9 is localized to a perivacuolar compartment. (A) Localization pattern of GFPCvt9. The _cvt9_Δ (AHY001) strain was transformed with a plasmid encoding a GFPCvt9 fusion protein behind the CUP1 promoter (pCuGFPCVT9). Expression of GFPCvt9 was induced with 10 μM CuSO4 for 2 h, followed by labeling of the vacuoles with FM 4-64 (see Materials and Methods). Images were taken with a Leica IRM confocal microscope. GFPCvt9 mostly localizes to intense perivacuolar, punctate structures. (B and C) Subcellular localization of Cvt9 by Optiprep density gradients. The _cvt9_Δ strain was transformed with pCuCVT9(416) and incubated with 50 μM CuSO4 at midlog growth stage to induce Cvt9 expression. The cells were converted to spheroplasts and lysed in gradient lysis buffer (see Materials and Methods). A total membrane fraction was isolated by centrifugation at 100,000 g for 20 min and loaded to the top of a 10-ml Optiprep linear gradient (0–66%). After centrifugation at 100,000 g for 16 h at 4°C, 14 fractions were collected and analyzed by immunoblots with antiserum or antibodies to Cvt9 and (B) Pho8 (vacuole), (C) Dpm1 (ER), Kex2 (trans-Golgi Network), and Pep12 (endosome). The immunoblots and the graphs of the densitometry quantitation are from the same gradient but presented separately for clarity.
Figure 4
Cvt9 is localized to a perivacuolar compartment. (A) Localization pattern of GFPCvt9. The _cvt9_Δ (AHY001) strain was transformed with a plasmid encoding a GFPCvt9 fusion protein behind the CUP1 promoter (pCuGFPCVT9). Expression of GFPCvt9 was induced with 10 μM CuSO4 for 2 h, followed by labeling of the vacuoles with FM 4-64 (see Materials and Methods). Images were taken with a Leica IRM confocal microscope. GFPCvt9 mostly localizes to intense perivacuolar, punctate structures. (B and C) Subcellular localization of Cvt9 by Optiprep density gradients. The _cvt9_Δ strain was transformed with pCuCVT9(416) and incubated with 50 μM CuSO4 at midlog growth stage to induce Cvt9 expression. The cells were converted to spheroplasts and lysed in gradient lysis buffer (see Materials and Methods). A total membrane fraction was isolated by centrifugation at 100,000 g for 20 min and loaded to the top of a 10-ml Optiprep linear gradient (0–66%). After centrifugation at 100,000 g for 16 h at 4°C, 14 fractions were collected and analyzed by immunoblots with antiserum or antibodies to Cvt9 and (B) Pho8 (vacuole), (C) Dpm1 (ER), Kex2 (trans-Golgi Network), and Pep12 (endosome). The immunoblots and the graphs of the densitometry quantitation are from the same gradient but presented separately for clarity.
Figure 5
Cvt9 physically associates with Apg1. The _apg1_Δ strain (YYK36) was transformed with a plasmid encoding myc-tagged Cvt9 (p[myc-CVT9]) with or without an additional plasmid encoding Apg1 (p[APG1]). The cell lysates were prepared as described in Materials and Methods and subjected to immunoprecipitation with anti-Apg1 antiserum (lanes 1, 3, and 5) or buffer only (lanes 2, 4, and 6). The immunoprecipitates (IP) were analyzed by immunoblot with antiserum to myc. The anti-Apg1–precipitated immunocomplex contains myc-tagged Cvt9 (lane 5).
Figure 6
Cvt9 and the P. pastoris homologue Gsa9 are required for peroxisome degradation. (A) The _S. cerevisiae cvt9_Δ strain is defective in pexophagy. Peroxisomes from wild-type (WT; KA311A) and _cvt9_Δ (YYK107) cells were proliferated in oleic acid medium and then degraded by shifting to SD-N medium at the indicated times (see Materials and Methods). Pexophagy was monitored by immunoblot analysis of the peroxisomal thiolase enzyme, Fox3. Fox3 levels decrease in wild-type cells under pexophagy conditions but remain constant in _cvt9_Δ. (B) The P. pastoris gsa9 strain is defective in pexophagy. Peroxisomes from wild-type (GS115), gsa7 (WDY7), and gsa9 (R8) cells were proliferated in methanol medium and then degraded by shifting to glucose medium for 0 and 6 h (see Materials and Methods). Pexophagy was measured by an activity assay for the peroxisomal AOX enzyme. AOX activity decreases in wild-type cells in glucose adaptation conditions, but remains relatively high in gsa7 and gsa9 cells. (C) The _P. pastoris gsa9_Δ strain is not defective in autophagy. Wild-type (GS115), gsa7 (WDY7), and gsa9 (R8) cells were radiolabeled with 1 μCi/ml [14C]valine for 16 h. The cells were then shifted to nitrogen-depleted chase medium and the production of TCA-soluble radioactivity was measured during 2–24 h of chase. Protein turnover rate in gsa9 cells is comparable to wild-type cells but relatively low in the autophagy-defective gsa7 cells. (D) The P. pastoris gsa9 mutants are defective at a middle to late stage of micropexophagy. Wild-type strain (GS115) and mutant strains gsa9-2 (R8) and _gsa9_Δ (WDK09) were grown to confluency under methanol conditions to proliferate peroxisomes. The strains were then shifted to a glucose-containing medium for 2 h to induce peroxisome degradation before fixation and processing for electron microscopy (see Materials and Methods). Partially degraded peroxisomes (Px) were found within the vacuole (V) of the parental GS115 cells. This was not observed in the gsa9-2 and _gsa9_Δ mutants. Instead, arm-like extensions of the vacuole(s) were observed to partially surround the peroxisomes. The nuclei (N) are also indicated.
Figure 7
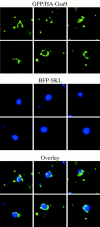
GFP/HA-Gsa9 localization during glucose adaptation. ANB23 cells expressing both BFP fused at its COOH terminus to the serine-lysine-leucine (SKL) peroxisomal targeting signal (BFP-SKL) and GFP/HA-Gsa9 were grown under methanol conditions, then adapted to glucose for 4 h. The peroxisomes were labeled by BFP-SKL (middle). During glucose adaptation, the GFP/HA-Gsa9 (top) was concentrated to regions of the vacuolar membrane that engulf the peroxisomes containing the BFP-SKL marker (see overlay in bottom panels). A punctate GFP/HA-Gsa9 pattern was also detected.
Similar articles
- Cvt18/Gsa12 is required for cytoplasm-to-vacuole transport, pexophagy, and autophagy in Saccharomyces cerevisiae and Pichia pastoris.
Guan J, Stromhaug PE, George MD, Habibzadegah-Tari P, Bevan A, Dunn WA Jr, Klionsky DJ. Guan J, et al. Mol Biol Cell. 2001 Dec;12(12):3821-38. doi: 10.1091/mbc.12.12.3821. Mol Biol Cell. 2001. PMID: 11739783 Free PMC article. - Cvt19 is a receptor for the cytoplasm-to-vacuole targeting pathway.
Scott SV, Guan J, Hutchins MU, Kim J, Klionsky DJ. Scott SV, et al. Mol Cell. 2001 Jun;7(6):1131-41. doi: 10.1016/s1097-2765(01)00263-5. Mol Cell. 2001. PMID: 11430817 Free PMC article. - Cargo proteins facilitate the formation of transport vesicles in the cytoplasm to vacuole targeting pathway.
Shintani T, Klionsky DJ. Shintani T, et al. J Biol Chem. 2004 Jul 16;279(29):29889-94. doi: 10.1074/jbc.M404399200. Epub 2004 May 11. J Biol Chem. 2004. PMID: 15138258 Free PMC article. - Autophagy, cytoplasm-to-vacuole targeting pathway, and pexophagy in yeast and mammalian cells.
Kim J, Klionsky DJ. Kim J, et al. Annu Rev Biochem. 2000;69:303-42. doi: 10.1146/annurev.biochem.69.1.303. Annu Rev Biochem. 2000. PMID: 10966461 Review. - Transport of proteins to the yeast vacuole: autophagy, cytoplasm-to-vacuole targeting, and role of the vacuole in degradation.
Teter SA, Klionsky DJ. Teter SA, et al. Semin Cell Dev Biol. 2000 Jun;11(3):173-9. doi: 10.1006/scdb.2000.0163. Semin Cell Dev Biol. 2000. PMID: 10906274 Review.
Cited by
- A novel fluorescence-activated cell sorting (FACS)-based screening identified ATG14, the gene required for pexophagy in the methylotrophic yeast.
Shiraishi K, Arima Y, Nakamura M, Nakatsuji T, Oku M, Sakai Y. Shiraishi K, et al. FEMS Yeast Res. 2024 Jan 9;24:foae022. doi: 10.1093/femsyr/foae022. FEMS Yeast Res. 2024. PMID: 39025789 Free PMC article. - Atg45 is an autophagy receptor for glycogen, a non-preferred cargo of bulk autophagy in yeast.
Isoda T, Takeda E, Hosokawa S, Hotta-Ren S, Ohsumi Y. Isoda T, et al. iScience. 2024 Apr 26;27(6):109810. doi: 10.1016/j.isci.2024.109810. eCollection 2024 Jun 21. iScience. 2024. PMID: 38832010 Free PMC article. - Glycogen Granules Are Degraded by Non-Selective Autophagy in Nitrogen-Starved Komagataella phaffii.
Wijewantha NV, Kumar R, Nazarko TY. Wijewantha NV, et al. Cells. 2024 Mar 7;13(6):467. doi: 10.3390/cells13060467. Cells. 2024. PMID: 38534311 Free PMC article. - A quantitative ultrastructural timeline of nuclear autophagy reveals a role for dynamin-like protein 1 at the nuclear envelope.
Mannino PJ, Perun A, Surovstev I, Ader NR, Shao L, Melia TJ, King MC, Lusk CP. Mannino PJ, et al. bioRxiv [Preprint]. 2024 Feb 15:2024.02.14.580336. doi: 10.1101/2024.02.14.580336. bioRxiv. 2024. PMID: 38405892 Free PMC article. Preprint. - The mitochondrial intermembrane space protein mitofissin drives mitochondrial fission required for mitophagy.
Fukuda T, Furukawa K, Maruyama T, Yamashita SI, Noshiro D, Song C, Ogasawara Y, Okuyama K, Alam JM, Hayatsu M, Saigusa T, Inoue K, Ikeda K, Takai A, Chen L, Lahiri V, Okada Y, Shibata S, Murata K, Klionsky DJ, Noda NN, Kanki T. Fukuda T, et al. Mol Cell. 2023 Jun 15;83(12):2045-2058.e9. doi: 10.1016/j.molcel.2023.04.022. Epub 2023 May 15. Mol Cell. 2023. PMID: 37192628 Free PMC article.
References
- Baba M., Osumi M., Ohsumi Y. Analysis of the membrane structures involved in autophagy in yeast by freeze-replica method. Cell Struct. Funct. 1995;20:465–471. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases