The neuronal repellent Slit inhibits leukocyte chemotaxis induced by chemotactic factors - PubMed (original) (raw)
The neuronal repellent Slit inhibits leukocyte chemotaxis induced by chemotactic factors
J Y Wu et al. Nature. 2001.
Abstract
Migration is a basic feature of many cell types in a wide range of species. Since the 1800s, cell migration has been proposed to occur in the nervous and immune systems, and distinct molecular cues for mammalian neurons and leukocytes have been identified. Here we report that Slit, a secreted protein previously known for its role of repulsion in axon guidance and neuronal migration, can also inhibit leukocyte chemotaxis induced by chemotactic factors. Slit inhibition of the chemokine-induced chemotaxis can be reconstituted by the co-expression of a chemokine receptor containing seven transmembrane domains and Roundabout (Robo), a Slit receptor containing a single transmembrane domain. Thus, there is a functional interaction between single and seven transmembrane receptors. Our results reveal the activity of a neuronal guidance cue in regulating leukocyte migration and indicate that there may be a general conservation of guidance mechanisms underlying metazoan cell migration. In addition, we have uncovered an inhibitor of leukocyte chemotaxis, and propose a new therapeutic approach to treat diseases involving leukocyte migration and chemotactic factors.
Figures
Figure 1
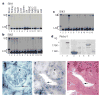
Expression of Slit and Robo in adult tissues. a–c, RNase protection assays (RPAs) were used to determine the expression of three slit genes in adult rats. Total RNA was prepared from different rat tissues and cell lines, as indicated (lanes 2–13). Total RNA (5 μg) was used in RPAs with probes specific for slit1, slit2 and slit3 genes (Sl). The rat L32 gene probe controlled for RNA input (lower band in each panel). Probes in lane 1 contain polylinker regions and are longer than the protected bands. LN, lymph node; PBMC, peripheral blood mononuclear cells; RAEC, rat endothelial cell line; GEDC, glomerular endothelial cells from the rat kidney; MC, rat kidney mesangial cells; 49F, rat kidney fibroblasts. d, Expression of Robo1 protein detected with anti-Robo1 antibodies. Arrowhead indicates full-length Robo1. Lane 1, HEK cells; lane 2, Robo1-transfected HEK cells; lane 3, a strong band with lower relative molecular mass (_M_r) than full-length Robo1 was detected reproducibly in rat thymus, indicating a crossreacting band or a proteolytically cleaved product; lane 4, HL-60 cells; lane 5, neutrophils differentiated from HL-60 cells; lane 6, rat PBMCs: no full-length Robo1 was detected but several lower _M_r bands were visible; lane 7, rat lymph node. e, slit2 messenger RNA distribution detected by in situ hybridization in the glomerulus of adult human kidney. Arrowhead indicates mesangial cells; arrow indicates epithelial cells in Bowman’s capsule. In the glomerulus, most of the positive cells are epithelial cells, but some endothelial cells can be seen. f, slit2 mRNA expression in vascular endothelial cells in the human kidney. Arrow indicates endothelial cells in a venule; arrowhead indicates endothelial cells in an arteriole. g, Haemotoxylin–eosin staining of a section of the same kidney as that shown in f. These two sections are not immediately next to each other, but corresponding regions are conveniently identified by landmarks in the sections.
Figure 2
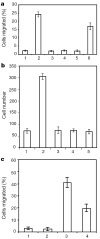
Effect of Slit on leukocyte chemotaxis induced by SDF-1α. a, Transwell migration of rat lymphocytes. Control or SDF-containing media were added to the lower chamber. Cells that migrated to the lower chamber were counted, and are expressed as a percentage of the cells added to the upper chamber. 1, control; 2, 10 nM SDF-1α; 3, 100 pM SLIT2; 4, 10 pM SLIT2; 5, 10 nM of SDF-1α and 100 pM of SLIT2; 6, 10 nM of SDF-1α and 10 pM of SLIT2. b, Rat lymphocytes were examined in transfilter assays in the presence of SDF-1α (10 nM). SLIT2 (100 pM) was added to the lower chamber, the upper chamber or both upper and lower chambers. 1, control; 2, SDF-1α; 3, both SDF-1α and SLIT2 in the lower chamber; 4, SDF-1α in the lower chamber and SLIT2 in the upper chamber; 5, SDF-1α in the lower chamber and SLIT2 in both chambers. c, Slit inhibition of chemotaxis induced by fMLP. HL-60 cells differentiated into neutrophil-like cells after treatment with DMSO. Chemotaxis was observed in transwell assays. 1, control; 2, SLIT2; 3, fMLP; 4, SLIT2 and fMLP.
Figure 3
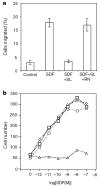
Robo is involved in mediating Slit inhibition of chemotaxis. a, The effect of Slit is inhibited by RoboN. Chemotaxis was analysed by using rat lymph node cells in transwell assays. SLIT2 (SL) and RoboN (RN) were added to the upper well and SDF-1α was added to the lower well. b, Expression of CXCR4 and Robo in HEK cells can reconstitute Slit inhibition of SDF-1α-induced chemotaxis. HEK migration was measured with the transfilter assay in microchemotaxis chambers using HEK cells expressing CXCR4, or both CXCR4 and rat Robo1 in the presence of different concentrations of SDF-1α. Control vehicle or 100 pM of purified Slit protein was added. Squares, migration of HEK cells expressing CXCR4 in response to SDF-1; diamonds, migration of HEK cells expressing CXCR4 in response to SDF-1α in the presence of Slit; circles, migration of HEK cells expressing Robo and CXCR4 in response to SDF-1α; triangles, migration of HEK cells expressing Robo and CXCR4 in response to SDF-1α in the presence of Slit.
Figure 4
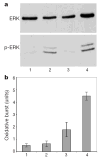
Lack of general inhibition by Slit. a, Neutrophils differentiated from HL-60 were treated with fMLP, or fMLP and SLIT2. Phosphorylation of ERK was examined by an anti-phospho-ERK antibody. Top, anti-ERK staining; bottom, anti-phospho-ERK staining. fMLP could induce ERK phosphorylation, which was not inhibited by the addition of SLIT2. Lane 1, control; lane 2, fMLP; lane 3, SLIT2; lane 4, fMLP and SLIT2. b, Oxidative burst was shown by SOD-inhibitable production of superoxide, and 1 unit was defined as nmol per 2 × 106 cells per 15 min. 1, control; 2, SLIT2; 3, fMLP; 4, SLIT 2 and fMLP. Results shown are representative of three experiments, each with triplicate samples.
Similar articles
- Slit-2/Robo-1 modulates the CXCL12/CXCR4-induced chemotaxis of T cells.
Prasad A, Qamri Z, Wu J, Ganju RK. Prasad A, et al. J Leukoc Biol. 2007 Sep;82(3):465-76. doi: 10.1189/jlb.1106678. Epub 2007 Jun 12. J Leukoc Biol. 2007. PMID: 17565045 Free PMC article. - Directional guidance of neuronal migration in the olfactory system by the protein Slit.
Wu W, Wong K, Chen J, Jiang Z, Dupuis S, Wu JY, Rao Y. Wu W, et al. Nature. 1999 Jul 22;400(6742):331-6. doi: 10.1038/22477. Nature. 1999. PMID: 10432110 Free PMC article. - Comparison of the roles of mitogen-activated protein kinase kinase and phosphatidylinositol 3-kinase signal transduction in neutrophil effector function.
Coffer PJ, Geijsen N, M'rabet L, Schweizer RC, Maikoe T, Raaijmakers JA, Lammers JW, Koenderman L. Coffer PJ, et al. Biochem J. 1998 Jan 1;329 ( Pt 1)(Pt 1):121-30. doi: 10.1042/bj3290121. Biochem J. 1998. PMID: 9405284 Free PMC article. - Slit: a roadblock for chemotaxis.
Fernandis AZ, Ganju RK. Fernandis AZ, et al. Sci STKE. 2001 Jul 17;2001(91):pe1. doi: 10.1126/stke.2001.91.pe1. Sci STKE. 2001. PMID: 11752663 Review. - Slit proteins: molecular guidance cues for cells ranging from neurons to leukocytes.
Wong K, Park HT, Wu JY, Rao Y. Wong K, et al. Curr Opin Genet Dev. 2002 Oct;12(5):583-91. doi: 10.1016/s0959-437x(02)00343-x. Curr Opin Genet Dev. 2002. PMID: 12200164 Review.
Cited by
- Enhancement of motor neuron development and function in zebrafish by sialyllacto-N-tetraose b.
Li P, Chen P, Zheng Y, Suo G, Shen F, Li H, Zhong X, Chen X, Wu Y. Li P, et al. Transl Pediatr. 2024 Jul 31;13(7):1201-1209. doi: 10.21037/tp-24-247. Epub 2024 Jul 29. Transl Pediatr. 2024. PMID: 39144427 Free PMC article. - Targeting Redundant ROBO1 and SDF-1 Pathways Prevents Adult Hemangioblast Derived-EPC and CEC Activity Effectively Blocking Tumor Neovascularization.
Shenoy AK, Pi L, Ligocki AP, Hosaka K, Cogle CR, Scott EW. Shenoy AK, et al. Stem Cell Rev Rep. 2023 May;19(4):928-941. doi: 10.1007/s12015-022-10498-7. Epub 2023 Jan 18. Stem Cell Rev Rep. 2023. PMID: 36652143 - Roles of axon guidance molecules in neuronal wiring in the developing spinal cord.
Chédotal A. Chédotal A. Nat Rev Neurosci. 2019 Jul;20(7):380-396. doi: 10.1038/s41583-019-0168-7. Nat Rev Neurosci. 2019. PMID: 31000796 Review. - Bisphenol A Activates an Innate Viral Immune Response Pathway.
Sowers ML, Tang H, Tian B, Goldblum R, Midoro-Horiuti T, Zhang K. Sowers ML, et al. J Proteome Res. 2020 Feb 7;19(2):644-654. doi: 10.1021/acs.jproteome.9b00548. Epub 2019 Dec 27. J Proteome Res. 2020. PMID: 31816243 Free PMC article. - Dendrite self-avoidance requires cell-autonomous slit/robo signaling in cerebellar purkinje cells.
Gibson DA, Tymanskyj S, Yuan RC, Leung HC, Lefebvre JL, Sanes JR, Chédotal A, Ma L. Gibson DA, et al. Neuron. 2014 Mar 5;81(5):1040-1056. doi: 10.1016/j.neuron.2014.01.009. Neuron. 2014. PMID: 24607227 Free PMC article.
References
- Devreotes PN, Zigmond SH. Chemotaxis in eukaryotic cells: a focus on leukocytes and Dictyostelium. Annu Rev Cell Biol. 1988;4:649–686. - PubMed
- McCutcheon M. Chemotaxis in leukocytes. Physiol Rev. 1946;26:319–336. - PubMed
- Bentivoglio M, Mazzarello P. The history of radial glia. Brain Res Bull. 1999;49:305–315. - PubMed
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. - PubMed
- Murphy PM. The molecular biology of leukocyte chemoattractant receptors. Annu Rev Immunol. 1994;12:593–633. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases