Proliferation of NG2-positive cells and altered oligodendrocyte numbers in the contused rat spinal cord - PubMed (original) (raw)
Proliferation of NG2-positive cells and altered oligodendrocyte numbers in the contused rat spinal cord
D M McTigue et al. J Neurosci. 2001.
Abstract
Given the numerous reparative roles glia may play after spinal cord injury (SCI), glial proliferation and cell number were examined in a model of traumatic SCI. Emphasis was placed on analysis of oligodendrocytes and NG2-positive (NG2+) cells, an endogenous cell population that may be involved in oligodendrocyte replacement. Overall, proliferation (assessed by bromodeoxyuridine incorporation) was markedly elevated during the first 2 weeks after injury and declined thereafter; a large portion of these dividing cells likely consisted of microglia-macrophages. Although the total number of NG2+ cells in the epicenter was reduced by half, we noted protracted proliferation in surviving NG2+ cells, with values sevenfold greater than in uninjured controls. Elevated proliferation of NG2+ cells persisted throughout the first 4 weeks after injury. However, the absolute number of NG2+ cells was not increased over controls, suggesting that the daughter cells either did not survive or they differentiated into other cell types. As expected, oligodendrocyte numbers were drastically altered after SCI. By 7 d after injury, the number of oligodendrocytes at the impact site was reduced by 93%. Despite ongoing tissue loss, the number of oligodendrocytes in spared tissue rose threefold at 14 d after injury. Although the function of NG2+ cells within the spinal cord is not completely understood, several studies suggest that they may differentiate into oligodendrocytes. Thus, proliferating NG2+ cells may contribute to the increased oligodendrocyte number observed at 2 weeks after injury. Future studies are required, however, to definitively determine the role NG2+ cells play in oligodendrocyte genesis, remyelination, and other post-injury events.
Figures
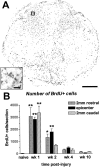
Fig. 1.
Distribution and time course of cell proliferation after spinal cord contusion injury. A, Epicenter section from 7 d post-injury spinal cord labeled immunohistochemically for BrdU. Note that most BrdU+ cells are found within the former dorsal horns and the central region of the cross-section. Scale bar, 0.4 mm.Inset, High-power view of the box in_A_. Several nuclei positively labeled for BrdU are visible. Scale bar, 20 μm. B, Quantification of total number of BrdU+ nuclei in cross-sections from the impact site and rostral and caudal regions of the lesion. Noninjured (naive) spinal cords contained minimal BrdU+ cells (30 cells per section). The number of dividing cells was significantly increased during the first and second weeks after injury at the epicenter. Proliferation also was elevated in rostral and caudal sections during the first week and in rostral sections during the second week after injury. Error bars represent means ± SEM. *p < 0.05; **p < 0.01.
Fig. 2.
NG2 labels progenitor cells and a subset of macrophages in the injured spinal cord. A, Example of NG2 immunostaining in a normal spinal cord (counterstained with neutral red). NG2+ progenitor cells are evenly distributed throughout the gray and white matter. The central canal is denoted with an asterisk. Scale bar, 50 μm. B, High-power view of the box from gray matter in_A_ illustrating cell soma and processes positively labeled for NG2 (arrow). Scale bar, 5 μm.C, Example of a cell (arrow) double-labeled for NG2 (gray) and BrdU (brown) from a section 2 mm rostral to the epicenter at 7 d after injury. A single-labeled BrdU+ nucleus is indicated by the arrowhead. Scale bar, 10 μm. D, Low-power view of a 28 d post-injury epicenter cross-section immunolabeled for NG2 and counterstained with neutral red. Increased NG2 immunoreactivity is present within the dorsal funiculus, central region, and spared white matter. Scale bar, 100 μm. E, High-power view of field within the_black box_ in D. A macrophage located within the lesion cavity with NG2 immunoreactivity present on the cell membrane is denoted with an arrow. Several nonlabeled macrophages are also visible. Scale bar: E,F, 10 μm. F, High-power view of field within the red box in D taken from the dorsal funiculus. An NG2+ macrophage (arrow) is visible within a region of increased NG2 deposition.
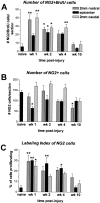
Fig. 3.
Quantification of NG2+ cells, NG2+/BrdU+ cells, and labeling index of dividing NG2+ cells within the epicenter and rostral and caudal regions of the lesion. A, Cells double-labeled for NG2 and BrdU were manually counted in cross-sections from the epicenter and 2 mm rostral and caudal after spinal contusion injury. An average of six double-labeled cells were detected in noninjured control tissue. After SCI, there was a significant increase in double-labeled cells during the first week after injury in rostral and caudal regions of the lesion, in all three regions during the second week, and in the caudal region during the fourth week after injury. B, All NG2+ cells in spinal cord cross-sections were manually counted. Approximately 140 cells per section were detected in spinal cord sections from normal animals. At 7 d after injury, NG2+ cell numbers were significantly reduced in the epicenter to 64 cells per section. This number increased to 106 cells per section at 14 d after injury and remained stable. At 10 weeks after injury, there were significantly fewer cells within sections 2 mm rostral to the impact site (67 cells per section). C, The labeling index of NG2+ cells was determined by dividing the number of cells double-labeled for NG2 and BrdU by the total number of NG2 cells for each animal. In naïve animals, ∼4% of NG2+ cells were double-labeled with BrdU, indicating a low turnover normally in this cell population. During the first week after injury, proliferation of these cells was significantly elevated within the epicenter and rostral and caudal regions of the lesion. This proliferation remained elevated within the epicenter until at least 4 weeks after injury and returned to baseline levels by 10 weeks after injury. Error bars represent means ± SEM. *p < 0.05; **p < 0.01.
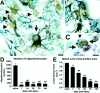
Fig. 4.
Proliferating cells in the injured spinal cord include microglia, macrophages, and astrocytes. Double-label immunohistochemistry for OX42 (microglia and macrophages;gray) and BrdU (brown;A–D), and GFAP (astrocytes; gray) and BrdU (brown; E, F).A, Epicenter cross-section at 7 d after injury. Most BrdU+ cells and OX42+ cells are located in the central region of the section in an overlapping manner. B, High-power view of the box in A. Several macrophages double-labeled with OX42 and BrdU are visible (arrows).Arrowheads indicate two macrophages without BrdU+ nuclei. Scale bar: B, F, 10 μm.C, Section 1 cm rostral to epicenter at 7 d after injury. Many activated microglia were visible, especially within the fasciculus gracilis (asterisk) and gray matter. Central canal is indicated by the arrow. Scale bar, 100 μm.D, High-power view of the box in_C_ showing a ramified microglial cell double-labeled with BrdU, indicating that microglial cells relatively long distances from the impact site respond rapidly to SCI. Scale bar, 5 μm.E, Epicenter cross-section at 7 d after injury. Most GFAP+ cells are located within the spared rim of tissue, whereas most BrdU+ cells are found centrally. F, High-power view of the box in E. One astrocyte double-labeled with GFAP (gray) and BrdU (brown) is denoted by the arrow.
Fig. 5.
Oligodendrocyte numbers are drastically reduced early but are maintained chronically in the injured spinal cord. A–C, Examples of triple-label immunohistochemistry for CC1 (brown), GFAP (black), and P0 (purple) used to count oligodendrocytes. Sections are from lesion epicenter at 28 d after injury and are counterstained with methyl green. Scale bars, 5 μm. A, Several single-labeled oligodendrocytes (brown; arrowheads) and one astrocyte (black; arrow) are visible. Image taken from lateral white matter. B, Example of GFAP+ astrocyte double-labeled with CC1. Image from dorsolateral edge of white matter.C, Image taken from lateral white matter in a region in which several Schwann cells with P0+ myelin were visible. In this field, two Schwann cells were clearly double-labeled with CC1 and P0 (arrows), whereas one Schwann cell was single-labeled for P0 (arrowhead). D, Quantification of oligodendrocytes single-labeled with CC1 in epicenter cross-sections. At 7 d after injury, oligodendrocytes were significantly reduced by 93% compared with sections taken from normal spinal cords at T8 (which contained 1165 ± 113 oligodendrocytes). The number of oligodendrocytes remained significantly lower than controls as late as 5 months after injury. However, between 7 and 14 d after injury, oligodendrocyte numbers increased more than threefold. E, Quantification of cross-sectional area of epicenter sections at different times after injury. Digitized sections were manually outlined and cavities were excluded such that only the remaining tissue area was measured. A continual loss of spinal cord tissue for at least 5 months after injury was detected.
Similar articles
- NG2 is a major chondroitin sulfate proteoglycan produced after spinal cord injury and is expressed by macrophages and oligodendrocyte progenitors.
Jones LL, Yamaguchi Y, Stallcup WB, Tuszynski MH. Jones LL, et al. J Neurosci. 2002 Apr 1;22(7):2792-803. doi: 10.1523/JNEUROSCI.22-07-02792.2002. J Neurosci. 2002. PMID: 11923444 Free PMC article. - Increase of NG2-positive cells associated with radial glia following traumatic spinal cord injury in adult rats.
Wu D, Shibuya S, Miyamoto O, Itano T, Yamamoto T. Wu D, et al. J Neurocytol. 2005 Dec;34(6):459-69. doi: 10.1007/s11068-006-8998-4. Epub 2006 Aug 10. J Neurocytol. 2005. PMID: 16902766 - Identity, distribution, and development of polydendrocytes: NG2-expressing glial cells.
Nishiyama A, Watanabe M, Yang Z, Bu J. Nishiyama A, et al. J Neurocytol. 2002 Jul-Aug;31(6-7):437-55. doi: 10.1023/a:1025783412651. J Neurocytol. 2002. PMID: 14501215 Review. - The reactions and role of NG2 glia in spinal cord injury.
Levine J. Levine J. Brain Res. 2016 May 1;1638(Pt B):199-208. doi: 10.1016/j.brainres.2015.07.026. Epub 2015 Jul 29. Brain Res. 2016. PMID: 26232070 Free PMC article. Review.
Cited by
- NG2-glia and their functions in the central nervous system.
Dimou L, Gallo V. Dimou L, et al. Glia. 2015 Aug;63(8):1429-51. doi: 10.1002/glia.22859. Epub 2015 May 24. Glia. 2015. PMID: 26010717 Free PMC article. Review. - Organotypic spinal cord slice culture to study neural stem/progenitor cell microenvironment in the injured spinal cord.
Kim HM, Lee HJ, Lee MY, Kim SU, Kim BG. Kim HM, et al. Exp Neurobiol. 2010 Sep;19(2):106-13. doi: 10.5607/en.2010.19.2.106. Epub 2010 Sep 30. Exp Neurobiol. 2010. PMID: 22110349 Free PMC article. - Endogenous proliferation after spinal cord injury in animal models.
McDonough A, Martínez-Cerdeño V. McDonough A, et al. Stem Cells Int. 2012;2012:387513. doi: 10.1155/2012/387513. Epub 2012 Dec 20. Stem Cells Int. 2012. PMID: 23316243 Free PMC article. - Progression in translational research on spinal cord injury based on microenvironment imbalance.
Fan B, Wei Z, Feng S. Fan B, et al. Bone Res. 2022 Apr 8;10(1):35. doi: 10.1038/s41413-022-00199-9. Bone Res. 2022. PMID: 35396505 Free PMC article. Review. - Oligodendrocyte fate after spinal cord injury.
Almad A, Sahinkaya FR, McTigue DM. Almad A, et al. Neurotherapeutics. 2011 Apr;8(2):262-73. doi: 10.1007/s13311-011-0033-5. Neurotherapeutics. 2011. PMID: 21404073 Free PMC article. Review.
References
- Adrian EK., Jr Cell division in injured spinal cord. Am J Anat. 1968;123:501–520. - PubMed
- Adrian EK, Jr, Walker BE. Incorporation of thymidine-H3 by cells in normal and injured mouse spinal cord. J Neuropathol Exp Neurol. 1962;21:597–609. - PubMed
- Adrian EK, Jr, Williams MG, George FC. Fine structure of reactive cells in injured nervous tissue labeled with 3H-thymidine injected before injury. J Comp Neurol. 1978;180:815–839. - PubMed
- Amat JA, Farooq M, Ishiguro H, Norton WT. Cells of the oligodendrocyte lineage proliferate following cortical stab wounds: an in vitro analysis. Glia. 1998;22:64–71. - PubMed
- Balentine JD. Pathology of experimental spinal cord trauma. II. Ultrastructure of axons and myelin. Lab Invest. 1978;39:254–266. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials