Negative regulation of Gcn4 and Msn2 transcription factors by Srb10 cyclin-dependent kinase - PubMed (original) (raw)
Negative regulation of Gcn4 and Msn2 transcription factors by Srb10 cyclin-dependent kinase
Y Chi et al. Genes Dev. 2001.
Abstract
The budding yeast transcriptional activator Gcn4 is rapidly degraded in an SCF(Cdc4)-dependent manner in vivo. Upon fractionation of yeast extracts to identify factors that mediate Gcn4 ubiquitination, we found that Srb10 phosphorylates Gcn4 and thereby marks it for recognition by SCF(Cdc4) ubiquitin ligase. Srb10 is a physiological regulator of Gcn4 stability because both phosphorylation and turnover of Gcn4 are diminished in srb10 mutants. Gcn4 is almost completely stabilized in srb10Delta pho85Delta cells, or upon mutation of all Srb10 phosphorylation sites within Gcn4, suggesting that the Pho85 and Srb10 cyclin-dependent kinases (CDKs) conspire to limit the accumulation of Gcn4. The multistress response transcriptional regulator Msn2 is also a substrate for Srb10 and is hyperphosphorylated in an Srb10-dependent manner upon heat-stress-induced translocation into the nucleus. Whereas Msn2 is cytoplasmic in resting wild-type cells, its nuclear exclusion is partially compromised in srb10 mutant cells. Srb10 has been shown to repress a subset of genes in vivo, and has been proposed to inhibit transcription via phosphorylation of the C-terminal domain of RNA polymerase II. We propose that Srb10 also inhibits gene expression by promoting the rapid degradation or nuclear export of specific transcription factors. Simultaneous down-regulation of both transcriptional regulatory proteins and RNA polymerase may enhance the potency and specificity of transcriptional inhibition by Srb10.
Figures
Figure 1
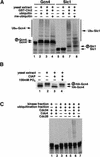
Gcn4 ubiquitination in yeast extracts requires SCF components and a protein kinase activity other than Cdc28. (A) [35S]Methionine-labeled Gcn4 (lanes 1_–_4) or Sic1 (lanes 5_–_8) synthesized by in vitro translation were incubated with G1-cyclin-depleted yeast whole cell extracts from RJD885 strain in the absence (lanes 2, 6) or the presence (lanes 3, 7) of purified GST–Cln2. Uba1, Cdc34, and SCF activities were supplied by the yeast extracts. Lanes 1 and 5 contained only input substrates. Methylated ubiquitin (me-ubiquitin) was used instead of wild-type ubiquitin in lanes 4 and 8. Encircled P refers to phosphorylated forms of Sic1 and Gcn4. All samples in panels A_–_C were evaluated by SDS-PAGE followed by autoradiography. (B) [35S]Methionine-labeled Gcn4HA translation product was phosphorylated by the DEAE flow-through fraction of RJD885 whole cell extract (see Materials and Methods). An aliquot of the reaction was immunoprecipitated using 12CA5 antibody and mock-treated (lane 2) or treated with calf intestinal alkaline phosphatase (CIAP) in the absence (lane 3) or the presence (lane 4) of phosphate inhibitor as described (Verma et al. 1997c). Immunoprecipitated input Gcn4HA is shown in lane 1. (C) Ubiquitination of Gcn4 in fractionated yeast extracts from a cdc4ts strain (RJD893). Extract fractions lacking Cdc34 but supplying either SCF components or a kinase activity were used (see Materials and Methods). A complete reaction (lane 6) included a kinase fraction (∼10 μg), a ubiquitination fraction (∼15 μg), Cdc34 (100 ng), and insect cell lysate containing baculovirus-expressed Cdc4 (∼4 μg total protein; Verma et al. 1997c). In lanes 1_–_5, one or more of the above components was omitted, as indicated. Insect cell lysate containing baculovirus-expressed Cdc28 was used as a specificity control (lane 7) for the Cdc4 lysate.
Figure 2
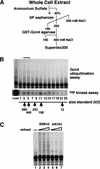
Identification of Srb10 as the protein kinase that promotes Gcn4 ubiquitination in yeast extracts. (A) Purification scheme (see Materials and Methods for details). (B) The indicated fractions from a Superdex 200 column were evaluated for both protein kinase activity using Gcn4 expressed in E. coli (bottom panel) and ubiquitination-promoting activity using [35S]methionine-labeled Gcn4 (top panel). Each ubiquitination reaction included ∼100 ng of triple infection SCFcdc4 (see Materials and Methods). The bracket highlights the peak ubiquitination-promoting activities (fractions 3 and 5). (C) Ubiquitination reactions were carried out with [35S]methionine-labeled Gcn4 (lane 1) in the presence of triple infection SCFcdc4 and a fraction of yeast extract that was enriched for Gcn4 kinase activity (see Materials and Methods). Varying amounts (5, 10, and 20 μg) of kinase fraction prepared from an SRB10 (Z719, lanes 2_–_4) or an srb10Δ (Z687, lanes 5_–_7) strain were tested.
Figure 3
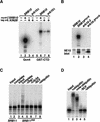
Srb10 directly phosphorylates Gcn4 and targets it for SCFCdc4-dependent ubiquitination. (A) Immunoprecipitates from SRB10myc9 strain YC6 (lanes 2, 6), KIN28myc9 strains YC15 (lanes 3, 7) and YC16 (lanes 4, 8), and untagged strain YC1 (lanes 1, 5) were assayed for kinase activity using, as substrate, Gcn4 (lanes 1_–_4) or GST–CTD (lanes 5_–_8) purified from E. coli. Reactions were evaluated by SDS-PAGE and autoradiography. (B) Same as A, except immunoprecipitates from SRB10myc9 strain YC17 (lane 2) and srb10-3myc9 strain YC7 (lane 4) were compared (top panel). Untagged strains Z719 (lane 1) and Z690 (lane 3) were used as controls. Antigen levels were monitored by immunoblotting duplicate immunoprecipitates with 9E10 antibody (bottom panel). (C) Srb10 protein kinase activity was immunoprecipitated from Z689 (SRB11HA) with 12CA5 antibody. This immunoprecipitate had strong Gcn4 kinase activity similar to the SRB10myc9 strains shown in A and B as judged by 32P kinase assays (data not shown). The ubiquitination assay was carried out by first incubating [35S]methionine-labeled Gcn4 with Srb11HA bound to protein A beads. An aliquot of the supernatant was used in the subsequent ubiquitination reactions (lanes 3_–_8). Immunoprecipitates from untagged strain Z719 were used as controls (lanes 1, 2). Ubiquitination reactions were either supplemented with a complete set of components (lanes 2, 4) including SCFCdc4 (triple infection) or a subset of components in which the factor indicated above each lane was omitted (lanes 5_–_8). (D) Gcn4 purified from E. coli was first phosphorylated in a kinase assay as shown in B, lane 2. 32P-Labeled Gcn4 was then added to ubiquitination reactions that were carried out as described in C, except SCFCdc4 (quadruple infection) was used.
Figure 4
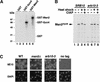
Srb10 phosphorylates Gcn4 and regulates its stability in vivo. (A) (Left panel) Cells from strains carrying untagged GCN4 (Z719, lane 1; Z687, lane 3; Z690, lane 5) or GCN4myc9 (YC19, lane 2; YC21, lane 4; YC45, lane 6) were pulse-labeled for 5 min (without chase) in SD medium containing Tran35Slabel. Cell lysates were immunoprecipitated using 9E10 antibody and evaluated by SDS-PAGE and autoradiography as described in Materials and Methods. (Right panel) Same as left panel, except the immunoprecipitated samples were either mock-treated (lanes 7, 9) or treated (lanes 8, 10) with 2 U CIAP at 37°C for 30 min. (B and D) Pulse-chase analysis of Gcn4myc9 stability in SRB10 (YC19), srb10-3 (YC45), CDC34 (YC19), and cdc34ts (YC35) cells. Quantitations of the experiments are shown on the right. The experiment in D was performed at 37°C. (C) SRB10 (Z719, lane 1; YC19, lanes 2, 3, 6, 7), srb10-3 (YC45, lanes 4, 5), or srb10Δ (YC21, lanes 8, 9) cells were cultured as described for the pulse-chase experiments, harvested, and lysed by boiling in SDS-containing buffer. Equal amounts (∼50 μg) of cell lysates were analyzed by immunoblotting using 9E10 (top panels) and anti-Cdc28 (bottom panels) antibodies. Each sample (except the untagged control) was run in duplicate lanes, and lanes 1_–_5 and 6_–_9 are from separate experiments. Bands were quantitated, and Gcn4 signals were normalized against Cdc28. Compared to wild type, the Gcn4 level is 1.7 times higher in srb10-3 and 2.0 times higher in srb10Δ cells.
Figure 5
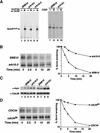
Both Srb10 and Pho85 contribute to Gcn4 turnover in vivo. (A) Gcn4myc9 stability under normal and overproduced conditions. Wild-type (YC19, YC23) and srb10Δ (YC21, YC24) cells grown in YP-raffinose medium were transferred either to synthetic galactose medium (top two panels) or synthetic dextrose medium (bottom two panels) for 1 h prior to being subjected to pulse-chase analysis as described for Figure 4A. Quantitation of the results is shown on the right. The arrowhead denotes a posttranslationally modified form of Gcn4myc9 that appeared rapidly in SRB10 cells, but was only slowly generated in srb10Δ strains. Note that the biphasic degradation observed for GAL–GCN4myc9 strains was likely owing to the carry-over synthesis of Gcn4myc9 during the first 5 min of chase. (B) Gcn4 stability in wild-type (YC19), srb10Δ (YC21), pho85Δ (YC59), and srb10Δ pho85Δ (YC61) cells was evaluated by pulse-chase analysis (see Fig. 4A legend). Quantitation is shown on the right. In two independent experiments, the half-life of Gcn4 in the various strains varied by less than 2 min.
Figure 6
Srb10-dependent degradation of Gcn4 is not regulated by amino acid starvation. (A) SRB10 (YC19) cells were grown to mid-log phase and transferred to either SD +leucine +histidine +uracil (− starvation) or SD −leucine +histidine +uracil (+ starvation) medium. Cells were cultured for 15 min, and pulse-chase analysis was carried out as described in Materials and Methods. Starving cells for 30 or 60 min resulted in similar magnitudes of stabilization of Gcn4 (data not shown). The half-life of Gcn4 (_t_1/2) was determined from quantitation of the data using Phosphorimager. (B) Same as A, except SRB10 (YC19, top panel) and srb10Δ (YC21, bottom panel) cells were assayed for Gcn4 stability in SD −leucine +histidine +uracil (+ starvation) cells only. (C) Same as A, except srb10Δ (YC21, top panels) cells and pho85Δ (YC59, bottom panels) cells were assayed for Gcn4 stability.
Figure 7
CDK phosphorylation is not essential for Gcn4 activity, but is required for its rapid degradation in vivo. (A) Strains with the indicated genotypes were grown on SD +leu (∼0.23 mM) +his +ura medium (control) and the same medium supplemented with 40 mM leucine (−Ile, −Val) to induce starvation for isoleucine and valine. Plates were photographed after incubation at 30°C for 3 d. (Top row of panels) Wild-type strain (Z719) and mutant strains srb10Δ (Z687), srb10-3 (Z690), and gcn4::HA3 (YC25). (Bottom row of panels) Strains containing _myc9_-tagged GCN4 alleles (YC19, YC21, YC45, YC57, and YC94). (B) Pulse-chase analysis (see Fig. 4A legend) of Gcn4myc9 stability in wild-type (YC19) and gcn4–3T2S (YC94) cells. Quantitation of the experiment is shown on the right.
Figure 8
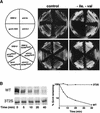
Msn2 is phosphorylated in an Srb10-dependent manner in vitro and in vivo, and its localization is influenced by Srb10. (A) Kinase assays were carried out with purified GST (lanes 1, 2), GST–Gcn4 (lanes 3, 4), and GST–Msn2 (lanes 5, 6) using immunoprecipitates from SRB10myc9 strain (YC17, lanes 2, 4, 6) and srb10-3myc9 strain (YC7, lanes 1, 3, 5) as described in Figure 3A. (B) Cells from MSN2myc9 (YC84, lanes 2_–_4) and MSN2myc9 srb10-3 (YC124, lanes 6_–_8) strains grown at 27°C were pulse-labeled with Tran35Slabel at 27°C for 5 min, and chased at 37°C for 5 min. Aliquots (1/3 before the heat shock and 2/3 after the heat shock) of the cultures were subjected to immunoprecipitation with 9E10 antibody. Immunoprecipitates from the heat shock samples were divided and either mock-treated (lane 3, 7) or treated (lanes 4, 8) with 2 U CIAP for 30 min at 37°C. Untagged strains Z719 (lane 1) and Z690 (lane 5) were used as controls. (C) Localization of Msn2myc9 in wild-type (YC84, a), _msn5_Δ (YC107, b), and srb10-3 (YC124, c) cells was evaluated under resting conditions by indirect immunofluorescence with 9E10 antibody. DAPI was used to stain cell nuclei. An srb10-3 strain containing untagged MSN2 (Z690) was used as control (d).
Comment in
- Transcriptional activation: risky business.
Tansey WP. Tansey WP. Genes Dev. 2001 May 1;15(9):1045-50. doi: 10.1101/gad.896501. Genes Dev. 2001. PMID: 11331599 Review.
Similar articles
- Degradation of the transcription factor Gcn4 requires the kinase Pho85 and the SCF(CDC4) ubiquitin-ligase complex.
Meimoun A, Holtzman T, Weissman Z, McBride HJ, Stillman DJ, Fink GR, Kornitzer D. Meimoun A, et al. Mol Biol Cell. 2000 Mar;11(3):915-27. doi: 10.1091/mbc.11.3.915. Mol Biol Cell. 2000. PMID: 10712509 Free PMC article. - GAL4 is regulated by the RNA polymerase II holoenzyme-associated cyclin-dependent protein kinase SRB10/CDK8.
Hirst M, Kobor MS, Kuriakose N, Greenblatt J, Sadowski I. Hirst M, et al. Mol Cell. 1999 May;3(5):673-8. doi: 10.1016/s1097-2765(00)80360-3. Mol Cell. 1999. PMID: 10360183 - Controlling transcription by destruction: the regulation of yeast Gcn4p stability.
Irniger S, Braus GH. Irniger S, et al. Curr Genet. 2003 Oct;44(1):8-18. doi: 10.1007/s00294-003-0422-3. Epub 2003 Jul 9. Curr Genet. 2003. PMID: 14508604 Review. - Transcriptional activation: risky business.
Tansey WP. Tansey WP. Genes Dev. 2001 May 1;15(9):1045-50. doi: 10.1101/gad.896501. Genes Dev. 2001. PMID: 11331599 Review.
Cited by
- The response to heat shock and oxidative stress in Saccharomyces cerevisiae.
Morano KA, Grant CM, Moye-Rowley WS. Morano KA, et al. Genetics. 2012 Apr;190(4):1157-95. doi: 10.1534/genetics.111.128033. Epub 2011 Dec 29. Genetics. 2012. PMID: 22209905 Free PMC article. Review. - Two cyclin-dependent kinases promote RNA polymerase II transcription and formation of the scaffold complex.
Liu Y, Kung C, Fishburn J, Ansari AZ, Shokat KM, Hahn S. Liu Y, et al. Mol Cell Biol. 2004 Feb;24(4):1721-35. doi: 10.1128/MCB.24.4.1721-1735.2004. Mol Cell Biol. 2004. PMID: 14749387 Free PMC article. - SCFCdc4-mediated degradation of the Hac1p transcription factor regulates the unfolded protein response in Saccharomyces cerevisiae.
Pal B, Chan NC, Helfenbaum L, Tan K, Tansey WP, Gething MJ. Pal B, et al. Mol Biol Cell. 2007 Feb;18(2):426-40. doi: 10.1091/mbc.e06-04-0304. Epub 2006 Nov 15. Mol Biol Cell. 2007. PMID: 17108329 Free PMC article. - Function and dynamics of the Mediator complex: novel insights and new frontiers.
Morse RH. Morse RH. Transcription. 2022 Feb-Jun;13(1-3):39-52. doi: 10.1080/21541264.2022.2085502. Epub 2022 Jun 16. Transcription. 2022. PMID: 35708525 Free PMC article. Review. - Fine-tuning of histone H3 Lys4 methylation during pseudohyphal differentiation by the CDK submodule of RNA polymerase II.
Law MJ, Ciccaglione K. Law MJ, et al. Genetics. 2015 Feb;199(2):435-53. doi: 10.1534/genetics.114.172841. Epub 2014 Dec 1. Genetics. 2015. PMID: 25467068 Free PMC article.
References
- Bai C, Sen P, Hofmann K, Ma L, Goebl M, Harper JW, Elledge SJ. SKP1 connects cell cycle regulators to the ubiquitin proteolysis machinery through a novel motif, the F-box. Cell. 1996;86:263–274. - PubMed
- Beck T, Hall MN. The TOR signalling pathway controls nuclear localization of nutrient-regulated transcription factors. Nature. 1999;402:689–692. - PubMed
- Carlson M. Genetics of transcriptional regulation in yeast: Connections to the RNA polymerase II CTD. Annu Rev Cell Dev Biol. 1997;13:1–23. - PubMed
- Carr SA, Huddleston MJ, Annan RS. Selective detection and sequencing of phosphopeptides at the femtomole level by mass spectrometry. Anal Biochem. 1996;239:180–192. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases