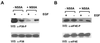Regulation of mRNA translation and cellular signaling by hepatitis C virus nonstructural protein NS5A - PubMed (original) (raw)
Regulation of mRNA translation and cellular signaling by hepatitis C virus nonstructural protein NS5A
Y He et al. J Virol. 2001 Jun.
Abstract
The NS5A nonstructural protein of hepatitis C virus (HCV) has been shown to inhibit the cellular interferon (IFN)-induced protein kinase R (PKR). PKR mediates the host IFN-induced antiviral response at least in part by inhibiting mRNA translation initiation through phosphorylation of the alpha subunit of eukaryotic initiation factor 2 (eIF2alpha). We thus examined the effect of NS5A inhibition of PKR on mRNA translation within the context of virus infection by using a recombinant vaccinia virus (VV)-based assay. The VV E3L protein is a potent inhibitor of PKR. Accordingly, infection of IFN-pretreated HeLa S3 cells with an E3L-deficient VV (VVDeltaE3L) resulted in increased phosphorylation levels of both PKR and eIF2alpha. IFN-pretreated cells infected with VV in which the E3L locus was replaced with the NS5A gene (VVNS5A) displayed diminished phosphorylation of PKR and eIF2alpha in a transient manner. We also observed an increase in activation of p38 mitogen-activated protein kinase in IFN-pretreated cells infected with VVDeltaE3L, consistent with reports that p38 lies downstream of the PKR pathway. Furthermore, these cells exhibited increased phosphorylation of the cap-binding initiation factor 4E (eIF4E), which is downstream of the p38 pathway. Importantly, these effects were reduced in cells infected with VVNS5A. NS5A was also found to inhibit activation of the p38-eIF4E pathway in epidermal growth factor-treated cells stably expressing NS5A. NS5A-induced inhibition of eIF2alpha and eIF4E phosphorylation may exert counteracting effects on mRNA translation. Indeed, IFN-pretreated cells infected with VVNS5A exhibited a partial and transient restoration of cellular and viral mRNA translation compared with IFN-pretreated cells infected with VVDeltaE3L. Taken together, these results support the role of NS5A as a PKR inhibitor and suggest a potential mechanism by which HCV might maintain global mRNA translation rate during early virus infection while favoring cap-independent translation of HCV mRNA during late infection.
Figures
FIG. 1
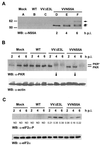
NS5A reduces virus-induced PKR autophosphorylation and eIF2α phosphoryltion. (A) Expression of NS5A in VVNS5A-infected cells. Lysates (20 μg of protein) from HeLa S3 cells pretreated with IFN-α/β and mock-infected (lane A) or infected with wild-type (WT) VV (lane B), VVΔE3L (lane C), or VVNS5A (lanes D through F) at 2, 4, or 6 h postinfection (p.i.) were resolved by SDS-PAGE (14% gel). For mock infections and infections with wild-type VV or VVΔE3L, only samples from the 6-h time point are shown. NS5A was detected by Western blotting (WB) using an anti-NS5A antibody (ID Labs). Arrows indicate the various migrating forms of NS5A. Sizes are indicated in kilodaltons. (B) Transient inhibition of virus-induced PKR autophosphorylation by NS5A. Lysates (20 μg of protein) used for panel A were resolved by SDS-PAGE (7.5% gel). PKR (top panel) and actin (bottom panel) proteins were detected with an anti-PKR antibody and an anti-actin (ICN) antibody, respectively. Hyperphosphorylated PKR protein is denoted by an asterisk. (C) Reduced eIF2α phosphorylation in the presence of NS5A. Lysates were immunoblotted with an antibody specific to the Ser51-phosphorylated form of eIF2α (top panel) or an anti-eIF2α antibody to detect protein levels (bottom panel). The relative levels of eIF2α phosphorylation were determined by quantitative densitometry and normalized against the individual total protein amounts. The ratio of the phospho-specific signal to the total protein signal is indicated below the lanes for lysates from VVΔE3L- and VVNS5A-infected cells. ND, not detectable.
FIG. 2
Inhibition of p38 activation and eIF4E phosphorylation by NS5A expression in VV-infected cells. (A) Virus-induced p38 activation in HeLa S3 cells infected with different recombinant VV at various time points postinfection (p.i.). Lysates (20 μg of protein) were fractionated by SDS-PAGE. The activation state of p38 was then determined by Western blotting (WB) using antibody specific for the dually phosphorylated activated form of p38 (Thr180/Tyr182) (top panel) or the total p38 protein kinase (bottom panel). The position of activated p38 is indicated by an arrow. The relative levels of p38 phosphorylation were determined by quantitative densitometry and normalized against the individual total protein amounts, and the phospho-specific signal/total protein signal ratios are indicated below the lanes for lysates from VVΔE3L- and VVNS5A-infected cells. ND, not detectable; WT, wild type. (B) Inhibition of Ser209 phosphorylation of eIF4E by NS5A expression in VV-infected cells. Phosphorylation levels of eIF4E at Ser209 were measured by Western blotting of the lysates used for Fig. 1 by using a phospho-specific antibody (top panel), while protein levels of eIF4E were determined by using an anti-eIF4E antibody (bottom panel). The relative levels of eIF4E phosphorylation were determined by quantitative densitometry and normalized against the individual total protein amounts. The ratio of the phospho-specific signal to total protein signal is indicated below the lanes.
FIG. 3
Inhibition of EGF-induced phosphorylation of p38 and eIF4E in an inducible stable NS5A-expressing HeLa cell line system. Tet-Off HeLa cells cultured in the presence (−NS5A) or in the absence of tetracycline (+NS5A) were mock- or EGF-treated (20 ng/ml for 2 min), washed, and incubated for 4 h prior to lysis. The activation state of p38 (A) and Ser209 phosphorylation of eIF4E (B) were then determined by immunoblotting using antibody directed against the phosphorylated forms of the proteins as described in the legend to Fig. 2. Total protein levels were determined as described in the legend to Fig. 2. WB, Western blotting.
FIG. 4
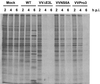
NS5A partially reverses the translational arrest phenotype observed in VVΔE3L-infected cells. (A) Profiles of global protein synthesis in recombinant VV-infected cells. HeLa S3 cells were pretreated with IFN-α/β and mock-infected or infected with wild-type (WT) VV, VVΔE3L, or VVNS5A. Cells were pulse-labeled with [35S]Met and harvested at 2, 4, and 6 h postinfection (p.i.) Lysates (20 μg of protein) were resolved by SDS-PAGE and detected by autoradiography. The protein translation levels were also quantified by subjecting lysates to TCA precipitation followed by scintillation counting. (B) Profiles of viral protein synthesis in recombinant VV-infected cells. The lysates used for panel A were immunoprecipitated with a rabbit polyclonal antiserum against total VV proteins, and precipitated proteins were subjected to SDS-PAGE and autoradiography.
FIG. 5
NS5A-mediated reversal of the translation arrest phenotype observed in VVΔE3L-infected cells is independent of its ability to bind Grb2. Analyses of global protein synthesis in HeLa S3 cells pretreated with IFN-α/β and infected with a recombinant VV expressing a Grb2 binding-defective form of NS5A (VVPro3) were performed as described in the legend to Fig. 4. WT, wild type; p.i., postinfection.
Similar articles
- Antiapoptotic and oncogenic potentials of hepatitis C virus are linked to interferon resistance by viral repression of the PKR protein kinase.
Gale M Jr, Kwieciszewski B, Dossett M, Nakao H, Katze MG. Gale M Jr, et al. J Virol. 1999 Aug;73(8):6506-16. doi: 10.1128/JVI.73.8.6506-6516.1999. J Virol. 1999. PMID: 10400746 Free PMC article. - Alpha interferon induces distinct translational control programs to suppress hepatitis C virus RNA replication.
Wang C, Pflugheber J, Sumpter R Jr, Sodora DL, Hui D, Sen GC, Gale M Jr. Wang C, et al. J Virol. 2003 Apr;77(7):3898-912. doi: 10.1128/jvi.77.7.3898-3912.2003. J Virol. 2003. PMID: 12634350 Free PMC article. - Control of PKR protein kinase by hepatitis C virus nonstructural 5A protein: molecular mechanisms of kinase regulation.
Gale M Jr, Blakely CM, Kwieciszewski B, Tan SL, Dossett M, Tang NM, Korth MJ, Polyak SJ, Gretch DR, Katze MG. Gale M Jr, et al. Mol Cell Biol. 1998 Sep;18(9):5208-18. doi: 10.1128/MCB.18.9.5208. Mol Cell Biol. 1998. PMID: 9710605 Free PMC article. - Repression of the PKR protein kinase by the hepatitis C virus NS5A protein: a potential mechanism of interferon resistance.
Gale MJ Jr, Korth MJ, Katze MG. Gale MJ Jr, et al. Clin Diagn Virol. 1998 Jul 15;10(2-3):157-62. doi: 10.1016/s0928-0197(98)00034-8. Clin Diagn Virol. 1998. PMID: 9741641 Review.
Cited by
- Vaccinia viruses with mutations in the E3L gene as potential replication-competent, attenuated vaccines: scarification vaccination.
Jentarra GM, Heck MC, Youn JW, Kibler K, Langland JO, Baskin CR, Ananieva O, Chang Y, Jacobs BL. Jentarra GM, et al. Vaccine. 2008 Jun 2;26(23):2860-72. doi: 10.1016/j.vaccine.2008.03.044. Epub 2008 Apr 8. Vaccine. 2008. PMID: 18455281 Free PMC article. - Complementation of vaccinia virus lacking the double-stranded RNA-binding protein gene E3L by human cytomegalovirus.
Child SJ, Jarrahian S, Harper VM, Geballe AP. Child SJ, et al. J Virol. 2002 May;76(10):4912-8. doi: 10.1128/jvi.76.10.4912-4918.2002. J Virol. 2002. PMID: 11967308 Free PMC article. - Identification of mutations in the HVR1 and PKR-BD regions in HCV-infected patients resistant to PEG-IFNα/RBV therapy.
Holysz M, Bialas K, Migdalski P, Kmieciak D, Trzeciak WH. Holysz M, et al. J Appl Genet. 2015 Aug;56(3):403-9. doi: 10.1007/s13353-014-0267-0. Epub 2015 Jan 15. J Appl Genet. 2015. PMID: 25588648 Free PMC article. - Blocking double-stranded RNA-activated protein kinase PKR by Japanese encephalitis virus nonstructural protein 2A.
Tu YC, Yu CY, Liang JJ, Lin E, Liao CL, Lin YL. Tu YC, et al. J Virol. 2012 Oct;86(19):10347-58. doi: 10.1128/JVI.00525-12. Epub 2012 Jul 11. J Virol. 2012. PMID: 22787234 Free PMC article. - Hepatitis C virus NS5A protein down-regulates the expression of spindle gene Aspm through PKR-p38 signaling pathway.
Wu SC, Chang SC, Wu HY, Liao PJ, Chang MF. Wu SC, et al. J Biol Chem. 2008 Oct 24;283(43):29396-404. doi: 10.1074/jbc.M802821200. Epub 2008 Aug 26. J Biol Chem. 2008. PMID: 18728014 Free PMC article.
References
- Alter M J H. Epidemiology of hepatitis C. Hepatology. 1997;26:62–65. - PubMed
- Barber G N, Tomita J, Garfinkel M S, Meurs E, Hovanessian A, Katze M G. Detection of protein kinase homologues and viral RNA-binding domains utilizing polyclonal antiserum prepared against a baculovirus-expressed dsRNA-activated 68,000-Da protein kinase. Virology. 1992;191:670–679. - PubMed
- Beattie E, Paoletti E, Tartaglia J. Distinct patterns of IFN sensitivity observed in cells infected with vaccinia K3L− and E3L− mutant viruses. Virology. 1995;210:254–263. - PubMed
- Beattie E, Tartaglia J, Paoletti E. Vaccinia virus-encoded eIF-2α homolog abrogates the antiviral effect of interferon. Virology. 1991;183:419–422. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P51 RR000166/RR/NCRR NIH HHS/United States
- RR-00166/RR/NCRR NIH HHS/United States
- AI-22646/AI/NIAID NIH HHS/United States
- R01 AI022646/AI/NIAID NIH HHS/United States
- AI-41629/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous