Tracking the spread of a lacZ-tagged herpes simplex virus type 1 between the eye and the nervous system of the mouse: comparison of primary and recurrent infection - PubMed (original) (raw)
Comparative Study
Tracking the spread of a lacZ-tagged herpes simplex virus type 1 between the eye and the nervous system of the mouse: comparison of primary and recurrent infection
C Shimeld et al. J Virol. 2001 Jun.
Abstract
The spread of herpes simplex virus type 1 (HSV-1) during primary ocular infection and after reactivation of latent infection in the trigeminal ganglion (TG) was examined in the mouse using a genetically modified virus containing the lacZ reporter gene under the control of the immediate-early 110 promoter. Whole tissue mounts of the eye and lids, their sensory nerves, and TG with the attached dorsal root entry zone (DRE) into the central nervous system (CNS) were stained for beta-galactosidase. Sixteen hours after inoculation of the cornea by scarification, staining was found in the scarified epithelium of the cornea and in the unscarified conjunctiva. By 24 h, staining was also seen in a few TG neurons and by 96 h their number had greatly increased and their distribution was more widespread. Stained cells (identified as Schwann cells by their staining for glial fibrillary acidic protein [GFAP] or S-100) in the TG were first seen close to stained neurons at 40 h, and by 48 h lines of such cells extended partway toward the periphery and toward the DRE. By 72 h, these lines had reached the periphery and the DRE where the adjacent CNS was also stained. In the cornea, stained cells with the morphology and arrangement of Schwann cells were seen from 40 to 120 h. After reactivation of latent infection, 10 of 22 samples had positively stained neurons. In eight samples, corneal and lid epithelial cells were stained. No stained Schwann cells were seen in the TG; however, branched networks of such cells were present in the cornea and the lids. This detailed sequential analysis has provided new information on the involvement of Schwann cells in the pathogenesis of primary and recurrent HSV-1 disease in the TG and the cornea.
Figures
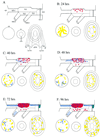
FIG. 1
Diagrammatic representation of β-Gal staining in whole tissue mounts (ocular structures, their trigeminal nerve supply, the trigeminal ganglion, and its root in the brain stem) at different times after inoculation of the cornea with HSV-1 SC16 IE110 lacZ. (A) Key to diagram. In the mounted specimen, the part containing the conjunctiva, lids, and skin (lower right in panel A) remains attached to the ophthalmic nerve, but for clarity this is drawn separately. Similarly, in the specimen, the iris (lower center in panel A) is a part of the anterior segment but again, for clarity, this is drawn separately. TG1, ophthalmic part of the trigeminal ganglion; TG2, maxillary part; TG3, mandibular part; oph. n, ophthalmic nerve; max. n, maxillary nerve; man. n, mandibular nerve; B.S, brainstem; d.r.e, dorsal root entry zone; cor, cornea; ir, iris; p.m., pupillary margin; sk, skin; l.m, lid margin; i.p. con, inferior palpebral conjunctiva; s.p. con, superior palpebral conjunctiva. In panels B through F, the colored areas indicate cells or regions with β-Gal staining: red, individual neuronal cell bodies (where such cells were too numerous to count, the region involved is represented as a larger red area); blue dashed lines, Schwann cells; yellow, areas of staining in epithelial tissues (cornea, conjunctiva, skin) and iris; blue-green, staining in the CNS.
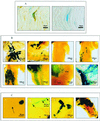
FIG. 2
(A) Histochemical detection of β-galactosidase and immunohistochemical detection of GFAP in mouse ophthalmic nerve 120 h after inoculation of the cornea with HSV-1 SC16 110 lacZ. Panel 1 shows a β-galactosidase (blue) and GFAP (brown) double-positive cell; panel 2 shows a β-galactosidase-positive cell stained as for panel 1, except that the anti-GFAP antibody was replaced by normal rabbit serum. (B) Histochemical detection of β-galactosidase in mouse tissues at different timepoints after similar inoculations (1 through 8). Expression was detected in the corneal epithelium (1) and the superior palpebral fornix of the conjunctiva (2) at 16 h after inoculation (the arrow marks nonspecific staining of meibomian glands), isolated neurons in the TG 24 h after infection (3), small groups of neurons, satellite cells, and Schwann cells in the TG 48 h after inoculation (4), large numbers of neurons, satellite cells and Schwann cells in the TG 72 h after infection (5), Schwann cells of the ophthalmic nerves 96 h after infection (6), the CNS at the DRE and Schwann cells in the PNS 120 h after infection (7) and the iris 120 h after inoculation (8) (arrow marks pupillary margin). (C) Histochemical detection of β-galactosidase in mouse tissues at different timepoints after reactivation of latent virus in the TG by UV irradiation of the cornea: a reactivating neuron in TG1 2 days after irradiation of the cornea (the arrow marks axonal staining) (1), a network of corneal nerve Schwann cells 2 days after UV irradiation (the inset shows apparent nuclear staining in Schwann cells [arrows], at higher magnification) (2), a dendritic lesion of the corneal epithelium 3 days after irradiation (3), and a lesion of the eyelid margin 3 days after irradiation (4).
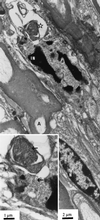
FIG. 3
Ophthalmic nerve 120 h after inoculation of HSV-1 SC16 IE110 lacZ. N, uninfected nucleus of myelinating Schwann cell; A, myelinated axon; IN, nucleus of unmyelinated Schwann cell with marginated chromatin. The inset shows area of infected nucleus at higher magnification. Arrows indicate virus capsids.
Similar articles
- Nectin-1-specific entry of herpes simplex virus 1 is sufficient for infection of the cornea and viral spread to the trigeminal ganglia.
Shukla ND, Tiwari V, Valyi-Nagy T. Shukla ND, et al. Mol Vis. 2012;18:2711-6. Epub 2012 Nov 16. Mol Vis. 2012. PMID: 23213272 Free PMC article. - Herpes simplex virus type 1 corneal infection results in periocular disease by zosteriform spread.
Summers BC, Margolis TP, Leib DA. Summers BC, et al. J Virol. 2001 Jun;75(11):5069-75. doi: 10.1128/JVI.75.11.5069-5075.2001. J Virol. 2001. PMID: 11333887 Free PMC article. - The herpes simplex virus type 1 ICP0 promoter is activated by viral reactivation stimuli in trigeminal ganglia neurons of transgenic mice.
Loiacono CM, Taus NS, Mitchell WJ. Loiacono CM, et al. J Neurovirol. 2003 Jun;9(3):336-45. doi: 10.1080/13550280390201047. J Neurovirol. 2003. PMID: 12775417 - The transgenic ICP4 promoter is activated in Schwann cells in trigeminal ganglia of mice latently infected with herpes simplex virus type 1.
Taus NS, Mitchell WJ. Taus NS, et al. J Virol. 2001 Nov;75(21):10401-8. doi: 10.1128/JVI.75.21.10401-10408.2001. J Virol. 2001. PMID: 11581408 Free PMC article. - [Battle with herpes for 37 years].
Shimomura Y. Shimomura Y. Nippon Ganka Gakkai Zasshi. 2015 Mar;119(3):145-66; discussion 167. Nippon Ganka Gakkai Zasshi. 2015. PMID: 25854108 Review. Japanese.
Cited by
- Microglia Activate Early Antiviral Responses upon Herpes Simplex Virus 1 Entry into the Brain to Counteract Development of Encephalitis-Like Disease in Mice.
Katzilieris-Petras G, Lai X, Rashidi AS, Verjans GMGM, Reinert LS, Paludan SR. Katzilieris-Petras G, et al. J Virol. 2022 Mar 23;96(6):e0131121. doi: 10.1128/JVI.01311-21. Epub 2022 Jan 19. J Virol. 2022. PMID: 35045263 Free PMC article. - Stress Induced Transcription Factors Transactivate the Herpes Simplex Virus 1 Infected Cell Protein 27 (ICP27) Transcriptional Enhancer.
Ostler JB, Jones C. Ostler JB, et al. Viruses. 2021 Nov 17;13(11):2296. doi: 10.3390/v13112296. Viruses. 2021. PMID: 34835102 Free PMC article. - Zinc oxide tetrapods inhibit herpes simplex virus infection of cultured corneas.
Duggal N, Jaishankar D, Yadavalli T, Hadigal S, Mishra YK, Adelung R, Shukla D. Duggal N, et al. Mol Vis. 2017 Feb 26;23:26-38. eCollection 2017. Mol Vis. 2017. PMID: 28275313 Free PMC article. - Impaired glucocorticoid receptor function attenuates herpes simplex virus 1 production during explant-induced reactivation from latency in female mice.
Harrison KS, Wijesekera N, Robinson AGJ, Santos VC, Oakley RH, Cidlowski JA, Jones C. Harrison KS, et al. J Virol. 2023 Oct 31;97(10):e0130523. doi: 10.1128/jvi.01305-23. Epub 2023 Oct 12. J Virol. 2023. PMID: 37823644 Free PMC article. - Analysis of herpes simplex virus ICP0 promoter function in sensory neurons during acute infection, establishment of latency, and reactivation in vivo.
Thompson RL, Shieh MT, Sawtell NM. Thompson RL, et al. J Virol. 2003 Nov;77(22):12319-30. doi: 10.1128/jvi.77.22.12319-12330.2003. J Virol. 2003. PMID: 14581568 Free PMC article.
References
- Blyth W A, Harbour D A, Hill T J. Pathogenesis of zosteriform spread of herpes simplex virus in the mouse. J Gen Virol. 1984;65:1477–1486. - PubMed
- Cook S D, Hill J M. Herpes simplex virus: molecular biology and the possibility of corneal latency. Surv Ophthalmol. 1991;36:140–148. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous