Deletion of the cytoplasmic tail of the fusion protein of the paramyxovirus simian virus 5 affects fusion pore enlargement - PubMed (original) (raw)
Deletion of the cytoplasmic tail of the fusion protein of the paramyxovirus simian virus 5 affects fusion pore enlargement
R E Dutch et al. J Virol. 2001 Jun.
Abstract
The fusion (F) protein of the paramxyovirus simian parainfluenza virus 5 (SV5) promotes virus-cell and cell-cell membrane fusion. Previous work had indicated that removal of the SV5 F protein cytoplasmic tail (F Tail- or FDelta19) caused a block in fusion promotion at the hemifusion stage. Further examination has shown that although the F Tail- mutant is severely debilitated in promotion of fusion as measured by using two reporter gene assays and is debilitated in the formation of syncytia relative to the wild-type F protein, the F Tail- mutant is capable of promoting the transfer of small aqueous dyes. These data indicate that F Tail- is fully capable of promoting formation of small fusion pores. However, enlargement of fusion pores is debilitated, suggesting that either the cytoplasmic tail of the F protein plays a direct role in pore expansion or that it interacts with other components which control pore growth.
Figures
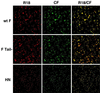
FIG. 1
Transfer of lipid and aqueous dyes by wt F and F Tail−. SV5 HN and either wt F or F Tail− were coexpressed in CV-1 cells by using the recombinant vac-T7 polymerase expression system. At 24 h p.t., human RBCs double labeled with the lipid probe R18 and aqueous probe CF were bound to CV-1 cells at 4°C. Fusion was initiated by replacement of cold PBS with PBS prewarmed to 37°C, and cells were incubated at 37°C for 10 min. Fusion was stopped by replacement with ice-cold PBS. Cells were examined using a confocal microscope (Zeiss LSM 410; Carl Zeiss, Inc., Thornwood, N.Y.), with dual images recorded on both fluorescein and rhodamine channels.
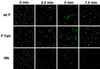
FIG. 2
Time course of transfer of the aqueous probe CF. Human RBCs labeled with the aqueous probe CF were bound to CV-1 cells coexpressing HN and either wt F or F Tail−, as described in the legend to Fig. 1. The cells were incubated at 37°C for various times, and the results were analyzed by confocal microscopy.
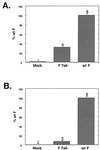
FIG. 3
Reporter gene assays of wt F and F Tail−. (A) β-Galactosidase assay. CV-1 cells infected with the vac-T7 polymerase recombinant and coexpressing HN and either wt F or F Tail− were incubated with a second population of CV-1 cells infected with wt vaccinia virus and transfected with the plasmid pINTT7 β-gal, which encodes β-galactosidase under the control of the T7 polymerase promoter. After incubation at 37°C for 4 h, samples were analyzed by a colorimetric lysate assay. Results shown are the average of triplicate samples and are representative of two separate experiments. (B) CAT assay. Vero cells were cotransfected with pCAGGS expressing HN and either wt F or F Tail−. In addition, these cells were transfected with the plasmid encoding CAT under control of the T7 polymerase promoter. A second set of Vero cells was transfected with pCAGGS expressing T7 polymerase. After overnight incubation, the T7 polymerase-expressing cells were overlaid on the cells expressing the F and HN proteins. Membrane fusion between the cell populations allows the T7 polymerase to transcribe the CAT gene, and subsequent CAT activity was assayed as described previously (14). Samples are an average of duplicates and are representative of three separate experiments.
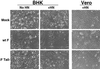
FIG. 4
Syncytium formation assay. BHK 21F cells or Vero cells were transfected using a total of 2 μg of pCAGGS wt F, F Tail−, or HN plasmid. At 18 to 24 h p.t. (BHK) or 36 h p.t. (Vero), monolayers were examined using a Nikon Diaphot inverted phase-contrast microscope (Nikon Inc., Garden City, N.Y.). Photographs were taken using a Kodak DCS 420 digital camera (Eastman Kodak Company, Rochester, N.Y.).
Similar articles
- Conserved glycine residues in the fusion peptide of the paramyxovirus fusion protein regulate activation of the native state.
Russell CJ, Jardetzky TS, Lamb RA. Russell CJ, et al. J Virol. 2004 Dec;78(24):13727-42. doi: 10.1128/JVI.78.24.13727-13742.2004. J Virol. 2004. PMID: 15564482 Free PMC article. - Mutations in multiple domains activate paramyxovirus F protein-induced fusion.
Seth S, Goodman AL, Compans RW. Seth S, et al. J Virol. 2004 Aug;78(16):8513-23. doi: 10.1128/JVI.78.16.8513-8523.2004. J Virol. 2004. PMID: 15280460 Free PMC article. - Roles for the cytoplasmic tails of the fusion and hemagglutinin-neuraminidase proteins in budding of the paramyxovirus simian virus 5.
Waning DL, Schmitt AP, Leser GP, Lamb RA. Waning DL, et al. J Virol. 2002 Sep;76(18):9284-97. doi: 10.1128/jvi.76.18.9284-9297.2002. J Virol. 2002. PMID: 12186912 Free PMC article. - The energetics of membrane fusion from binding, through hemifusion, pore formation, and pore enlargement.
Cohen FS, Melikyan GB. Cohen FS, et al. J Membr Biol. 2004 May 1;199(1):1-14. doi: 10.1007/s00232-004-0669-8. J Membr Biol. 2004. PMID: 15366419 Review. - Osmotic phenomena in membrane fusion.
Lucy JA. Lucy JA. Biochem Soc Trans. 1989 Aug;17(4):623-4. doi: 10.1042/bst0170623. Biochem Soc Trans. 1989. PMID: 2670629 Review. No abstract available.
Cited by
- Characterization of a structural intermediate of flavivirus membrane fusion.
Stiasny K, Kössl C, Lepault J, Rey FA, Heinz FX. Stiasny K, et al. PLoS Pathog. 2007 Feb;3(2):e20. doi: 10.1371/journal.ppat.0030020. PLoS Pathog. 2007. PMID: 17305426 Free PMC article. - The EFF-1A Cytoplasmic Domain Influences Hypodermal Cell Fusions in C. elegans But Is Not Dependent on 14-3-3 Proteins.
Shinn-Thomas JH, del Campo JJ, Wang J, Mohler WA. Shinn-Thomas JH, et al. PLoS One. 2016 Jan 22;11(1):e0146874. doi: 10.1371/journal.pone.0146874. eCollection 2016. PLoS One. 2016. PMID: 26800457 Free PMC article. - The actin cytoskeleton inhibits pore expansion during PIV5 fusion protein-promoted cell-cell fusion.
Wurth MA, Schowalter RM, Smith EC, Moncman CL, Dutch RE, McCann RO. Wurth MA, et al. Virology. 2010 Aug 15;404(1):117-26. doi: 10.1016/j.virol.2010.04.024. Virology. 2010. PMID: 20537366 Free PMC article. - Genetic control of fusion pore expansion in the epidermis of Caenorhabditis elegans.
Gattegno T, Mittal A, Valansi C, Nguyen KC, Hall DH, Chernomordik LV, Podbilewicz B. Gattegno T, et al. Mol Biol Cell. 2007 Apr;18(4):1153-66. doi: 10.1091/mbc.e06-09-0855. Epub 2007 Jan 17. Mol Biol Cell. 2007. PMID: 17229888 Free PMC article.
References
- Baker K A, Dutch R E, Lamb R A, Jardetzky T S. Structural basis for paramyxovirus-mediated membrane fusion. Mol Cell. 1999;3:309–319. - PubMed
- Bullough P A, Hughson F M, Skehel J J, Wiley D C. Structure of influenza haemagglutinin at the pH of membrane fusion. Nature. 1994;371:37–43. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources