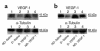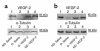Reversal of experimental diabetic neuropathy by VEGF gene transfer - PubMed (original) (raw)
Reversal of experimental diabetic neuropathy by VEGF gene transfer
P Schratzberger et al. J Clin Invest. 2001 May.
Abstract
The pathogenetic basis for diabetic neuropathy has been enigmatic. Using two different animal models of diabetes, we have investigated the hypothesis that experimental diabetic neuropathy results from destruction of the vasa nervorum and can be reversed by administration of an angiogenic growth factor. Nerve blood flow, as measured by laser Doppler imaging or direct detection of a locally administered fluorescent lectin analogue, was markedly attenuated in rats with streptozotocin-induced diabetes, consistent with a profound reduction in the number of vessels observed. A severe peripheral neuropathy developed in parallel, characterized by significant slowing of motor and sensory nerve conduction velocities, compared with nondiabetic control animals. In contrast, 4 weeks after intramuscular gene transfer of plasmid DNA encoding VEGF-1 or VEGF-2, vascularity and blood flow in the nerves of treated animals were similar to those of nondiabetic control rats; constitutive overexpression of both transgenes resulted in restoration of large and small fiber peripheral nerve function. Similar experiments performed in a rabbit model of alloxan-induced diabetes produced comparable results. These findings support the notion that diabetic neuropathy results from microvascular ischemia involving the vasa nervorum and suggest the feasibility of a novel treatment strategy for patients in whom peripheral neuropathy constitutes a secondary complication of diabetes.
Figures
Figure 1
Time course of MCV (a) and SCV (b). Female Sprague-Dawley rats weighing 240–260 g were injected intraperitoneally with streptozotocin at 50 mg/kg. Diabetes was confirmed by testing blood glucose the next day. Twelve weeks later, nondiabetic and diabetic rats were randomly assigned to a saline-injection control group, and to the treatment groups that underwent either VEGF-1 or VEGF-2 gene transfer (nondiabetic, saline injection, n = 5; nondiabetic, VEGF-1, n = 6; nondiabetic, VEGF-2, n = 5; diabetic, saline-injection, n = 9; diabetic, VEGF-1, n = 18; and diabetic, VEGF-2, n = 15). Sciatic nerve conduction measurements were performed at the time of treatment (week 0) and then at 2, 4, and 10 weeks. Data are expressed as mean ± SEM. A_P_ < 0.01, nondiabetic saline versus diabetic saline, diabetic VEGF-1, or diabetic VEGF-2. BDiabetic VEGF-1 or diabetic VEGF-2 not significant versus nondiabetic saline. C_P_ < 0.01, diabetic saline versus diabetic VEGF-1, diabetic VEGF-2, or nondiabetic. D_P_ < 0.01, diabetic saline versus diabetic VEGF-1, diabetic VEGF-2, or nondiabetic. (c) Tailflick threshold temperatures 4 weeks after gene transfer. The temperature at which the rats demonstrated the characteristic tailflick response was tested for in a completely blinded fashion. Values are expressed as mean ± SEM. A_P_ < 0.01. Nondiabetic, saline-injection, n = 5; diabetic, saline-injection, n = 9; diabetic, VEGF-1, n = 18; and diabetic, VEGF-2, n = 15.
Figure 2
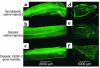
Representative fluorescence photomicrographs of longitudinal views of whole-mounted rat sciatic nerves (a–c) and their respective paraffin-embedded cross sections (d–f) 4 weeks after treatment. Before sacrifice and harvesting of the nerves, in vivo perfusion with FITC-conjugated BS-1 lectin, an endothelial-specific ligand, was performed. (a and d) Samples taken from a nondiabetic saline-injected control animal, showing a normal pattern of vascularity. (b and e) Samples taken from a diabetic animal 12 weeks after induction of diabetes and 4 weeks after sham treatment (saline injection). The total network of vasa nervorum is markedly reduced, resulting in an irregular distribution pattern and areas of nonvascularized nerve tissue. Note in particular the reduction of stained endoneurial vessels in the cross-sectional image. (c and f) Samples from a rat after 12 weeks of diabetes and 4 weeks after VEGF-1 gene transfer. Vascularity appears well preserved, and the number of visible vessels in the cross section appears similar to that of a normal sciatic nerve. ×2 (a–c); ×4 (d–f).
Figure 3
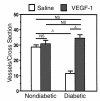
Quantification of vessels in tissue cross sections of rat sciatic nerve. Before sacrifice at 4 weeks after treatment (gene transfer or saline injections), animals were perfused with FITC-conjugated BS-1 lectin to visualize vasa nervorum. Ten cross sections per paraffin-embedded specimen were randomly selected from each specimen, and vessels per cross section were counted under a fluorescence microscope (×4) by an investigator blinded to treatment group. Data are expressed as mean ± SEM (n = 5 per study group), where individual numbers per animal were averaged from right and left sciatic nerves. A_P_ < 0.01.
Figure 4
In vivo LDPI of blood flow in rat sciatic nerve 4 weeks after gene transfer or saline injection. Nerves were surgically exposed from the sciatic notch to the knee level before three repeated LDPI measurements were obtained from the region of interest. (a) Bar graph (mean ± SEM) summarizes results of LDPI measurements taken from both sides of five rats per study group. A_P_ < 0.01 versus nondiabetic saline-injected. B_P_ < 0.01 versus diabetic saline-injected. (b) Representative color-coded LDPI. Lowest blood flow is indicated in blue, maximum blood flow in red, and intermediate grading in green and yellow. Nondiabetic saline-injected: perfusion of sciatic nerve in a normal, age-matched rat that underwent saline injection. Diabetic saline-injected: markedly reduced perfusion of sciatic nerve in a diabetic rat, 4 weeks after saline injection. Diabetic VEGF gene transfer: substantial restoration of sciatic nerve perfusion in a diabetic rat 4 weeks after VEGF gene transfer.
Figure 5
Expression of VEGF protein in nondiabetic and diabetic (12 weeks after streptozotocin treatment) rats. Western blot analysis of VEGF-1 protein expression in hindlimb muscles (a) and sciatic nerves (b) harvested 2 weeks after sham (saline) or gene injections. The level of VEGF protein expression was significantly reduced in diabetic versus nondiabetic rats. VEGF-1 gene transfer led to restoration of VEGF-1 protein expression both in muscles and in nerves of diabetic rats. Lane 1: nondiabetic saline-injected. Lane 2: diabetic saline-injected. Lane 3: diabetic, VEGF-1 gene transfer. Lane 4: nondiabetic, VEGF-1 gene transfer. VEGF-1 is detected as a 44-kDa protein. Western blots were reprobed with an anti–α-tubulin antibody to confirm equal protein loading of lanes. Similar results were obtained in three additional experiments.
Figure 6
Representative fluorescence photomicrographs of longitudinal views of whole-mounted rabbit nerves (tibial portion of the sciatic nerve) (a–c) and their respective paraffin-embedded cross sections (d–f) 8 weeks after treatment. Before sacrifice and harvesting of the nerves, in vivo perfusion with FITC-conjugated BS-1 lectin, an endothelial-specific ligand, was performed. (a and d) Samples from a nondiabetic saline-injected control animal, showing a regular pattern of vascularity. (b and e) Samples taken from a diabetic animal 6 months after induction of diabetes and 8 weeks after sham treatment (saline injection). The total network of vasa nervorum is markedly reduced, resulting in an irregular distribution pattern. Note the reduction of stained endoneurial vessels in the cross section. (c and f) Samples from a rabbit after 6 months of diabetes and 8 weeks after VEGF-2 gene transfer. The vascularity appears well preserved, and the number of vessels visible in the cross section appears similar to that of a normal sciatic nerve. ×2 (a–c); ×4 (d–f).
Figure 7
Representative color-coded images of in vivo LDPI in rabbit sciatic nerve 8 weeks after VEGF-2 gene transfer or saline injection. Nerves were surgically exposed from the sciatic notch to the knee level before three repeated LDPI measurements were obtained. Lowest blood flow is indicated in blue, maximum blood flow in red, and intermediate grading in green and yellow. Nondiabetic saline-injected: perfusion of sciatic nerve in a normal, age-matched rabbit that underwent saline injection. Diabetic saline-injected: markedly reduced perfusion of sciatic nerve in a diabetic rabbit, 8 weeks after sham saline injection. Diabetic VEGF-2 gene transfer: substantial restoration of sciatic nerve perfusion in a diabetic rabbit 8 weeks after VEGF gene transfer.
Figure 8
Expression of VEGF protein in nondiabetic and diabetic (6 months after alloxan treatment) rabbits. Western blot analysis of VEGF-2 protein expression in hindlimb muscles (a) and sciatic nerves (b) harvested 2 weeks after sham (saline) or gene injections. Lane 1: nondiabetic, saline injected. Lane 2: diabetic saline-injected. Lane 3: diabetic, VEGF-2 gene transfer. Lane 4: nondiabetic, VEGF-2 gene transfer. Molecular masses are indicated at the right. The precursor form of VEGF-2 is detected as a 58-kDa protein. The level of VEGF-2 protein expression was significantly reduced in diabetic compared with nondiabetic rabbits. VEGF-2 gene transfer led to a restoration of VEGF-2 protein expression in both muscles and nerves of diabetic rabbits that was similar to expression in nondiabetic animals. Western blots were reprobed with an anti–α-tubulin antibody to confirm equal protein loading of lanes. Similar results were obtained in four additional experiments.
Comment in
- Can VEGF reverse diabetic neuropathy in human subjects?
Veves A, King GL. Veves A, et al. J Clin Invest. 2001 May;107(10):1215-8. doi: 10.1172/JCI13038. J Clin Invest. 2001. PMID: 11375408 Free PMC article. No abstract available.
Similar articles
- VEGF gene transfer for diabetic neuropathy.
Isner JM, Ropper A, Hirst K. Isner JM, et al. Hum Gene Ther. 2001 Aug 10;12(12):1593-4. Hum Gene Ther. 2001. PMID: 11529248 Clinical Trial. - Sonic hedgehog induces arteriogenesis in diabetic vasa nervorum and restores function in diabetic neuropathy.
Kusano KF, Allendoerfer KL, Munger W, Pola R, Bosch-Marce M, Kirchmair R, Yoon YS, Curry C, Silver M, Kearney M, Asahara T, Losordo DW. Kusano KF, et al. Arterioscler Thromb Vasc Biol. 2004 Nov;24(11):2102-7. doi: 10.1161/01.ATV.0000144813.44650.75. Epub 2004 Sep 9. Arterioscler Thromb Vasc Biol. 2004. PMID: 15358602 - Antiangiogenesis mediates cisplatin-induced peripheral neuropathy: attenuation or reversal by local vascular endothelial growth factor gene therapy without augmenting tumor growth.
Kirchmair R, Walter DH, Ii M, Rittig K, Tietz AB, Murayama T, Emanueli C, Silver M, Wecker A, Amant C, Schratzberger P, Yoon YS, Weber A, Panagiotou E, Rosen KM, Bahlmann FH, Adelman LS, Weinberg DH, Ropper AH, Isner JM, Losordo DW. Kirchmair R, et al. Circulation. 2005 May 24;111(20):2662-70. doi: 10.1161/CIRCULATIONAHA.104.470849. Epub 2005 May 16. Circulation. 2005. PMID: 15897348 - VEGF gene therapy for coronary artery disease and peripheral vascular disease.
Rasmussen HS, Rasmussen CS, Macko J. Rasmussen HS, et al. Cardiovasc Radiat Med. 2002 Apr-Jun;3(2):114-7. doi: 10.1016/s1522-1865(02)00158-0. Cardiovasc Radiat Med. 2002. PMID: 12699842 Review. - Nerve and ganglion blood flow in diabetes: an appraisal.
Zochodne DW. Zochodne DW. Int Rev Neurobiol. 2002;50:161-202. doi: 10.1016/s0074-7742(02)50077-5. Int Rev Neurobiol. 2002. PMID: 12198810 Review.
Cited by
- Bone Marrow-Derived Mesenchymal Stem Cells Improve Diabetic Neuropathy by Direct Modulation of Both Angiogenesis and Myelination in Peripheral Nerves.
Han JW, Choi D, Lee MY, Huh YH, Yoon YS. Han JW, et al. Cell Transplant. 2016;25(2):313-26. doi: 10.3727/096368915X688209. Epub 2015 May 13. Cell Transplant. 2016. PMID: 25975801 Free PMC article. - Can VEGF reverse diabetic neuropathy in human subjects?
Veves A, King GL. Veves A, et al. J Clin Invest. 2001 May;107(10):1215-8. doi: 10.1172/JCI13038. J Clin Invest. 2001. PMID: 11375408 Free PMC article. No abstract available. - Effect of cartilage oligomeric matrix protein angiopoietin-1 on peripheral nerves in db/db diabetic mice.
Jin HY, Piao MH, Park JH, Baek HS, Lee S, Kim W, Park SK, Kim CH, Koh GY, Park TS. Jin HY, et al. Curr Ther Res Clin Exp. 2008 Aug;69(4):343-55. doi: 10.1016/j.curtheres.2008.08.002. Curr Ther Res Clin Exp. 2008. PMID: 24692811 Free PMC article. - Immune dysregulation in patients with carpal tunnel syndrome.
Moalem-Taylor G, Baharuddin B, Bennett B, Krishnan AV, Huynh W, Kiernan MC, Shin-Yi Lin C, Shulruf B, Keoshkerian E, Cameron B, Lloyd A. Moalem-Taylor G, et al. Sci Rep. 2017 Aug 15;7(1):8218. doi: 10.1038/s41598-017-08123-6. Sci Rep. 2017. PMID: 28811623 Free PMC article. - Paracrine control of vascularization and neurogenesis by neurotrophins.
Emanueli C, Schratzberger P, Kirchmair R, Madeddu P. Emanueli C, et al. Br J Pharmacol. 2003 Oct;140(4):614-9. doi: 10.1038/sj.bjp.0705458. Epub 2003 Aug 26. Br J Pharmacol. 2003. PMID: 12970083 Free PMC article. Review.
References
- Tomlinson DR, Fernyhough P, Diemel LT. Role of neurotrophins in diabetic neuropathy and treatment with nerve growth factors. Diabetes. 1997;46:S43–S49. - PubMed
- Reiber, G.E., Boyko, E.J., and Smith, D.G. 1995. Lower extremity foot ulcers and amputations in diabetes. In Diabetes in America. M.I. Harris et al., editors. National Institute of Diabetes and Digestive and Kidney Diseases. Washington, DC, USA. 409–427.
- Parkhouse N, LeQuesne PM. Impaired neurogenic vascular response in patients with diabetes and neuropathic foot lesions. N Engl J Med. 1988;318:1306–1309. - PubMed
- Veves A, et al. Endothelial dysfunction and the expression of endothelial nitric oxide synthase in diabetic neuropathy, vascular disease, and foot ulceration. Diabetes. 1998;47:457–463. - PubMed
- The Diabetes ControlComplications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–988. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL057516/HL/NHLBI NIH HHS/United States
- HL57516/HL/NHLBI NIH HHS/United States
- R37 HL053354/HL/NHLBI NIH HHS/United States
- HL53354/HL/NHLBI NIH HHS/United States
- HL60911/HL/NHLBI NIH HHS/United States
- R01 HL053354/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical